

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Retinoic acid receptor beta (Homo sapiens (Human)) | BDBM323588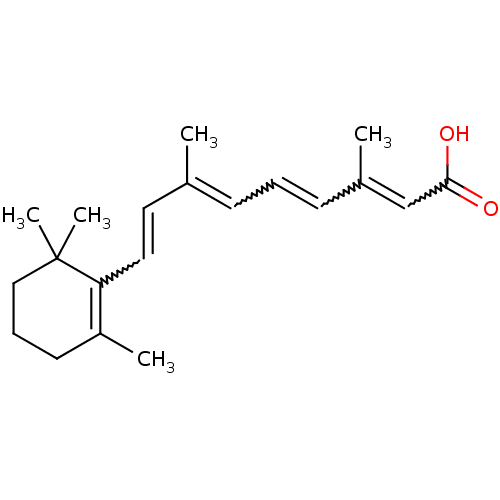 (Retinoic Acid | US10188615, at-RA | US10752616, Co...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1.88 | n/a | n/a | n/a | n/a |
King''s College London US Patent | Assay Description Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... | US Patent US10752616 (2020) BindingDB Entry DOI: 10.7270/Q2B85C6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM323588 (Retinoic Acid | US10188615, at-RA | US10752616, Co...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a |
King''s College London US Patent | Assay Description Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... | US Patent US10752616 (2020) BindingDB Entry DOI: 10.7270/Q2B85C6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor gamma (Homo sapiens (Human)) | BDBM323588 (Retinoic Acid | US10188615, at-RA | US10752616, Co...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a |
King''s College London US Patent | Assay Description Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... | US Patent US10752616 (2020) BindingDB Entry DOI: 10.7270/Q2B85C6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor beta (Homo sapiens (Human)) | BDBM50526314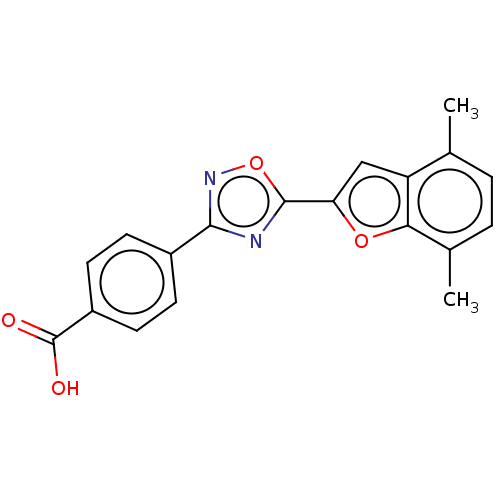 (CHEMBL4459692 | US10752616, Code No. BHBA-001) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1.94 | n/a | n/a | n/a | n/a |
King''s College London US Patent | Assay Description Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... | US Patent US10752616 (2020) BindingDB Entry DOI: 10.7270/Q2B85C6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM50526314 (CHEMBL4459692 | US10752616, Code No. BHBA-001) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 26 | n/a | n/a | n/a | n/a |
King''s College London US Patent | Assay Description Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... | US Patent US10752616 (2020) BindingDB Entry DOI: 10.7270/Q2B85C6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor gamma (Homo sapiens (Human)) | BDBM50526314 (CHEMBL4459692 | US10752616, Code No. BHBA-001) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
King''s College London US Patent | Assay Description Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... | US Patent US10752616 (2020) BindingDB Entry DOI: 10.7270/Q2B85C6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor beta (Homo sapiens (Human)) | BDBM458121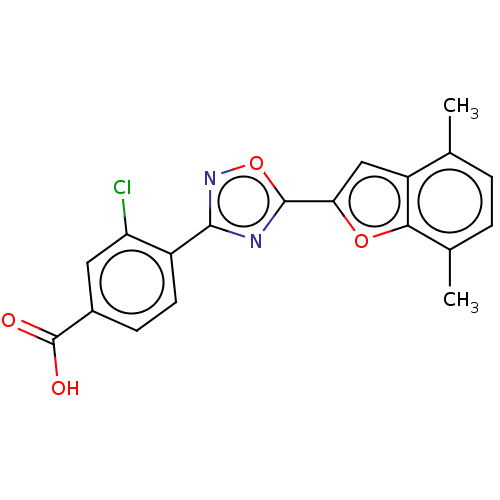 (US10752616, Code No. BHBA-002) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 11.4 | n/a | n/a | n/a | n/a |
King''s College London US Patent | Assay Description Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... | US Patent US10752616 (2020) BindingDB Entry DOI: 10.7270/Q2B85C6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM458121 (US10752616, Code No. BHBA-002) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 136 | n/a | n/a | n/a | n/a |
King''s College London US Patent | Assay Description Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... | US Patent US10752616 (2020) BindingDB Entry DOI: 10.7270/Q2B85C6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor gamma (Homo sapiens (Human)) | BDBM458121 (US10752616, Code No. BHBA-002) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 29 | n/a | n/a | n/a | n/a |
King''s College London US Patent | Assay Description Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... | US Patent US10752616 (2020) BindingDB Entry DOI: 10.7270/Q2B85C6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor beta (Homo sapiens (Human)) | BDBM458124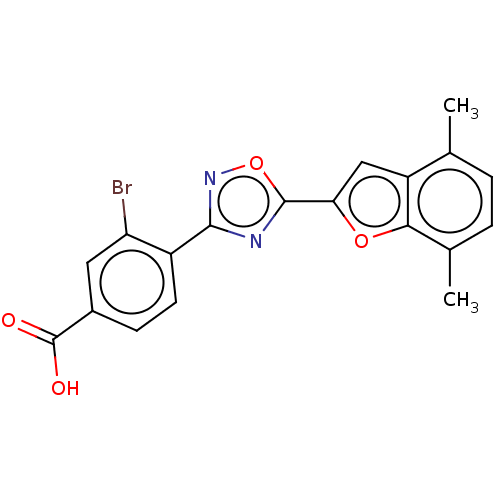 (US10752616, Code No. BHBA-003) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a |
King''s College London US Patent | Assay Description Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... | US Patent US10752616 (2020) BindingDB Entry DOI: 10.7270/Q2B85C6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM458124 (US10752616, Code No. BHBA-003) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 160 | n/a | n/a | n/a | n/a |
King''s College London US Patent | Assay Description Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... | US Patent US10752616 (2020) BindingDB Entry DOI: 10.7270/Q2B85C6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor gamma (Homo sapiens (Human)) | BDBM458124 (US10752616, Code No. BHBA-003) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 36 | n/a | n/a | n/a | n/a |
King''s College London US Patent | Assay Description Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... | US Patent US10752616 (2020) BindingDB Entry DOI: 10.7270/Q2B85C6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor beta (Homo sapiens (Human)) | BDBM458127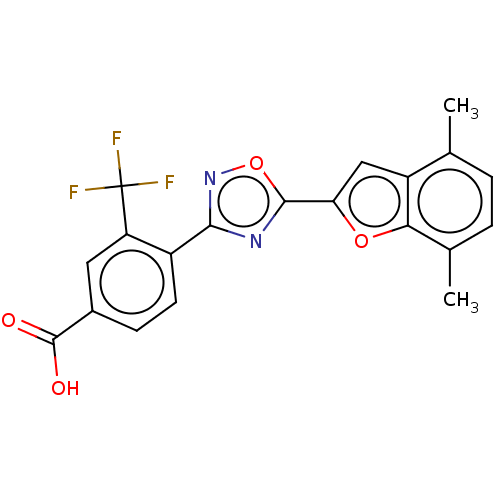 (US10752616, Code No. BHBA-004) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
King''s College London US Patent | Assay Description Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... | US Patent US10752616 (2020) BindingDB Entry DOI: 10.7270/Q2B85C6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor beta (Homo sapiens (Human)) | BDBM458133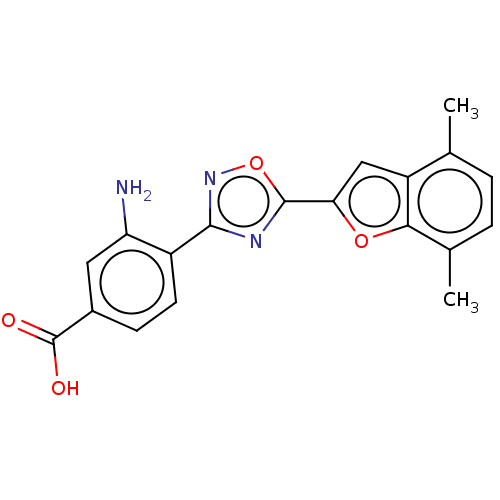 (US10752616, Code No. BHBA-005) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 23 | n/a | n/a | n/a | n/a |
King''s College London US Patent | Assay Description Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... | US Patent US10752616 (2020) BindingDB Entry DOI: 10.7270/Q2B85C6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM458133 (US10752616, Code No. BHBA-005) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 130 | n/a | n/a | n/a | n/a |
King''s College London US Patent | Assay Description Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... | US Patent US10752616 (2020) BindingDB Entry DOI: 10.7270/Q2B85C6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor gamma (Homo sapiens (Human)) | BDBM458133 (US10752616, Code No. BHBA-005) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 55 | n/a | n/a | n/a | n/a |
King''s College London US Patent | Assay Description Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... | US Patent US10752616 (2020) BindingDB Entry DOI: 10.7270/Q2B85C6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor beta (Homo sapiens (Human)) | BDBM50526313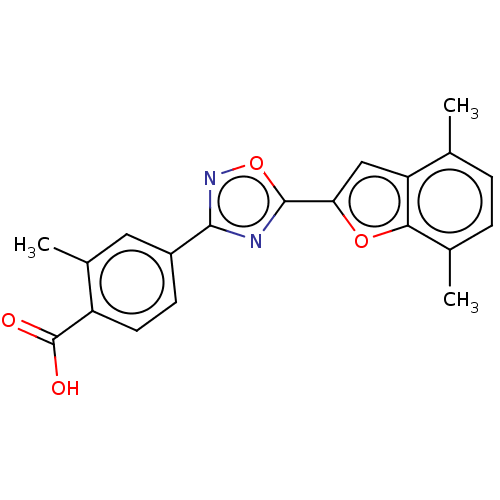 (CHEMBL4469143 | US10752616, Code No. BHBA-006) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
King''s College London US Patent | Assay Description Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... | US Patent US10752616 (2020) BindingDB Entry DOI: 10.7270/Q2B85C6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM50526313 (CHEMBL4469143 | US10752616, Code No. BHBA-006) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 89 | n/a | n/a | n/a | n/a |
King''s College London US Patent | Assay Description Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... | US Patent US10752616 (2020) BindingDB Entry DOI: 10.7270/Q2B85C6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor gamma (Homo sapiens (Human)) | BDBM50526313 (CHEMBL4469143 | US10752616, Code No. BHBA-006) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
King''s College London US Patent | Assay Description Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... | US Patent US10752616 (2020) BindingDB Entry DOI: 10.7270/Q2B85C6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor beta (Homo sapiens (Human)) | BDBM50526312 (CHEMBL4449668 | US10752616, Code No. BHBA-007) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a |
King''s College London US Patent | Assay Description Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... | US Patent US10752616 (2020) BindingDB Entry DOI: 10.7270/Q2B85C6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM50526312 (CHEMBL4449668 | US10752616, Code No. BHBA-007) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a |
King''s College London US Patent | Assay Description Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... | US Patent US10752616 (2020) BindingDB Entry DOI: 10.7270/Q2B85C6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor gamma (Homo sapiens (Human)) | BDBM50526312 (CHEMBL4449668 | US10752616, Code No. BHBA-007) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a |
King''s College London US Patent | Assay Description Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... | US Patent US10752616 (2020) BindingDB Entry DOI: 10.7270/Q2B85C6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor beta (Homo sapiens (Human)) | BDBM458145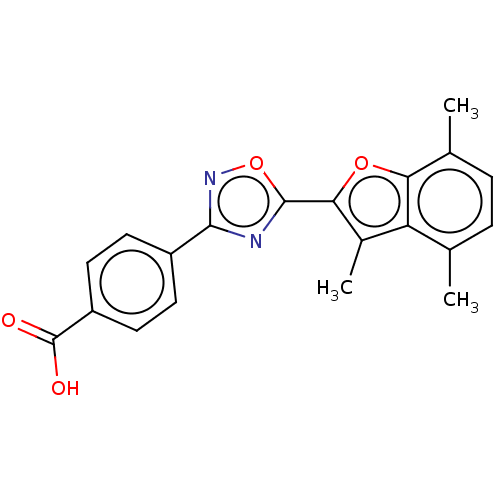 (US10752616, Code No. BHBA-008) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a |
King''s College London US Patent | Assay Description Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... | US Patent US10752616 (2020) BindingDB Entry DOI: 10.7270/Q2B85C6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM458145 (US10752616, Code No. BHBA-008) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a |
King''s College London US Patent | Assay Description Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... | US Patent US10752616 (2020) BindingDB Entry DOI: 10.7270/Q2B85C6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor gamma (Homo sapiens (Human)) | BDBM458145 (US10752616, Code No. BHBA-008) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a |
King''s College London US Patent | Assay Description Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... | US Patent US10752616 (2020) BindingDB Entry DOI: 10.7270/Q2B85C6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor beta (Homo sapiens (Human)) | BDBM458150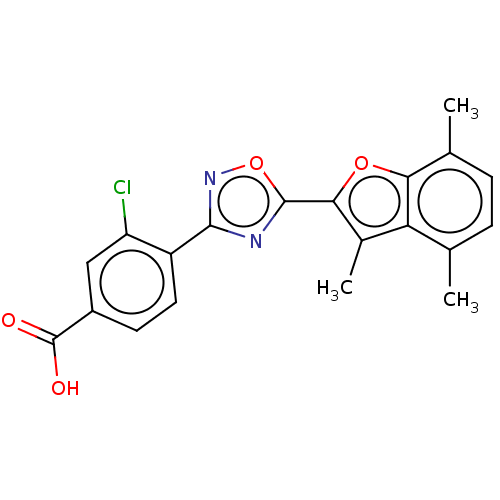 (US10752616, Code No. BHBA-009) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a |
King''s College London US Patent | Assay Description Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... | US Patent US10752616 (2020) BindingDB Entry DOI: 10.7270/Q2B85C6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM458150 (US10752616, Code No. BHBA-009) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 32 | n/a | n/a | n/a | n/a |
King''s College London US Patent | Assay Description Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... | US Patent US10752616 (2020) BindingDB Entry DOI: 10.7270/Q2B85C6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor gamma (Homo sapiens (Human)) | BDBM458150 (US10752616, Code No. BHBA-009) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
King''s College London US Patent | Assay Description Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... | US Patent US10752616 (2020) BindingDB Entry DOI: 10.7270/Q2B85C6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor beta (Homo sapiens (Human)) | BDBM458152 (US10752616, Code No. BHBA-010) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a |
King''s College London US Patent | Assay Description Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... | US Patent US10752616 (2020) BindingDB Entry DOI: 10.7270/Q2B85C6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM458152 (US10752616, Code No. BHBA-010) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
King''s College London US Patent | Assay Description Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... | US Patent US10752616 (2020) BindingDB Entry DOI: 10.7270/Q2B85C6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor gamma (Homo sapiens (Human)) | BDBM458152 (US10752616, Code No. BHBA-010) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a |
King''s College London US Patent | Assay Description Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... | US Patent US10752616 (2020) BindingDB Entry DOI: 10.7270/Q2B85C6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor beta (Homo sapiens (Human)) | BDBM458158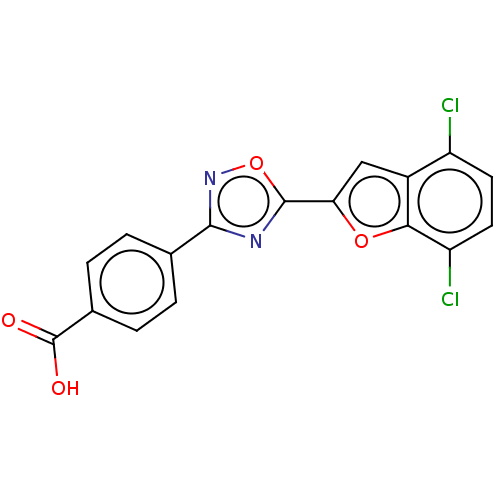 (US10752616, Code No. BHBA-011) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a |
King''s College London US Patent | Assay Description Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... | US Patent US10752616 (2020) BindingDB Entry DOI: 10.7270/Q2B85C6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM458158 (US10752616, Code No. BHBA-011) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 26 | n/a | n/a | n/a | n/a |
King''s College London US Patent | Assay Description Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... | US Patent US10752616 (2020) BindingDB Entry DOI: 10.7270/Q2B85C6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor gamma (Homo sapiens (Human)) | BDBM458158 (US10752616, Code No. BHBA-011) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
King''s College London US Patent | Assay Description Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... | US Patent US10752616 (2020) BindingDB Entry DOI: 10.7270/Q2B85C6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor beta (Homo sapiens (Human)) | BDBM458165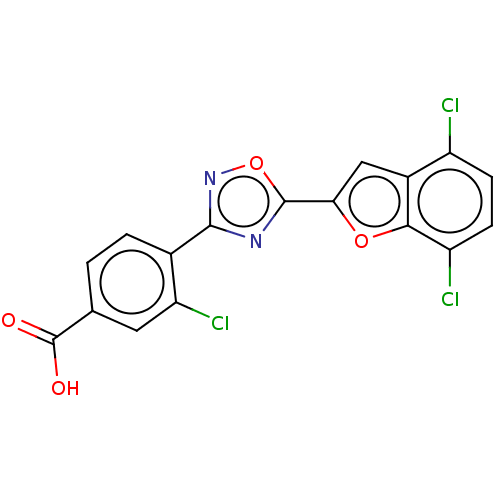 (US10752616, Code No. BHBA-012) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a |
King''s College London US Patent | Assay Description Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... | US Patent US10752616 (2020) BindingDB Entry DOI: 10.7270/Q2B85C6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM458165 (US10752616, Code No. BHBA-012) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 67 | n/a | n/a | n/a | n/a |
King''s College London US Patent | Assay Description Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... | US Patent US10752616 (2020) BindingDB Entry DOI: 10.7270/Q2B85C6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor gamma (Homo sapiens (Human)) | BDBM458165 (US10752616, Code No. BHBA-012) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a |
King''s College London US Patent | Assay Description Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... | US Patent US10752616 (2020) BindingDB Entry DOI: 10.7270/Q2B85C6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor beta (Homo sapiens (Human)) | BDBM458201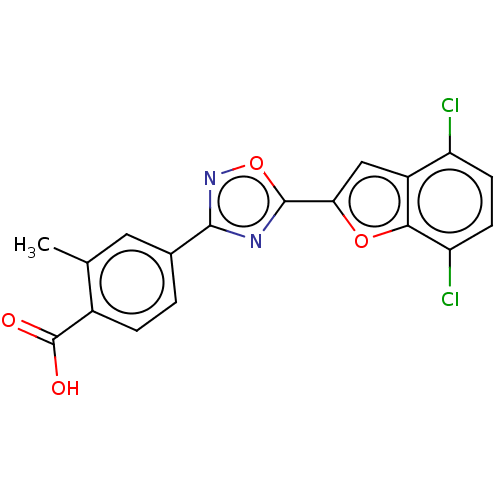 (US10752616, Code No. BHBA-013) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a |
King''s College London US Patent | Assay Description Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... | US Patent US10752616 (2020) BindingDB Entry DOI: 10.7270/Q2B85C6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM458201 (US10752616, Code No. BHBA-013) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 140 | n/a | n/a | n/a | n/a |
King''s College London US Patent | Assay Description Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... | US Patent US10752616 (2020) BindingDB Entry DOI: 10.7270/Q2B85C6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor gamma (Homo sapiens (Human)) | BDBM458201 (US10752616, Code No. BHBA-013) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a |
King''s College London US Patent | Assay Description Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... | US Patent US10752616 (2020) BindingDB Entry DOI: 10.7270/Q2B85C6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor beta (Homo sapiens (Human)) | BDBM458233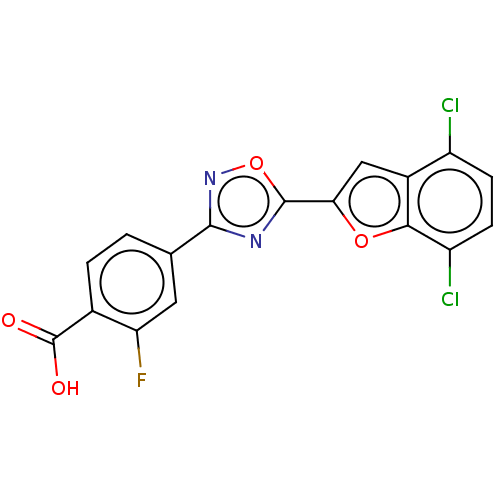 (US10752616, Code No. BHBA-014) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
King''s College London US Patent | Assay Description Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... | US Patent US10752616 (2020) BindingDB Entry DOI: 10.7270/Q2B85C6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM458233 (US10752616, Code No. BHBA-014) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
King''s College London US Patent | Assay Description Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... | US Patent US10752616 (2020) BindingDB Entry DOI: 10.7270/Q2B85C6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor gamma (Homo sapiens (Human)) | BDBM458233 (US10752616, Code No. BHBA-014) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
King''s College London US Patent | Assay Description Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... | US Patent US10752616 (2020) BindingDB Entry DOI: 10.7270/Q2B85C6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor beta (Homo sapiens (Human)) | BDBM458240 (US10752616, Code No. BHBA-015) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a |
King''s College London US Patent | Assay Description Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... | US Patent US10752616 (2020) BindingDB Entry DOI: 10.7270/Q2B85C6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM458240 (US10752616, Code No. BHBA-015) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 23 | n/a | n/a | n/a | n/a |
King''s College London US Patent | Assay Description Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... | US Patent US10752616 (2020) BindingDB Entry DOI: 10.7270/Q2B85C6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor gamma (Homo sapiens (Human)) | BDBM458240 (US10752616, Code No. BHBA-015) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
King''s College London US Patent | Assay Description Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... | US Patent US10752616 (2020) BindingDB Entry DOI: 10.7270/Q2B85C6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor beta (Homo sapiens (Human)) | BDBM458251 (US10752616, Code No. BHBA-016) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a |
King''s College London US Patent | Assay Description Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... | US Patent US10752616 (2020) BindingDB Entry DOI: 10.7270/Q2B85C6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM458251 (US10752616, Code No. BHBA-016) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 18 | n/a | n/a | n/a | n/a |
King''s College London US Patent | Assay Description Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... | US Patent US10752616 (2020) BindingDB Entry DOI: 10.7270/Q2B85C6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor gamma (Homo sapiens (Human)) | BDBM458251 (US10752616, Code No. BHBA-016) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
King''s College London US Patent | Assay Description Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... | US Patent US10752616 (2020) BindingDB Entry DOI: 10.7270/Q2B85C6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor beta (Homo sapiens (Human)) | BDBM458259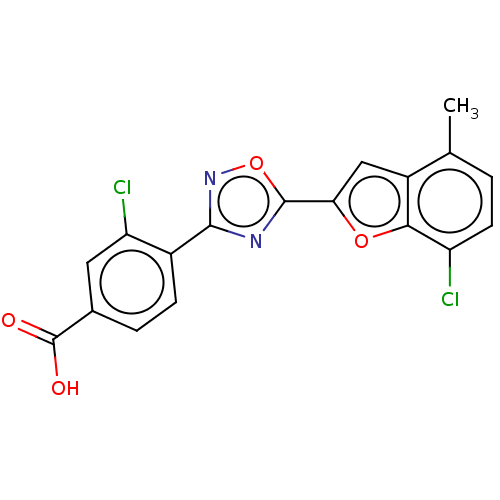 (US10752616, Code No. BHBA-017) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a |
King''s College London US Patent | Assay Description Transcriptional transactivation assays were performed with gal4 fusion receptor constructs, created using each of the RAR ligand binding domains of e... | US Patent US10752616 (2020) BindingDB Entry DOI: 10.7270/Q2B85C6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 136 total ) | Next | Last >> |