

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ceramide glucosyltransferase (Homo sapiens (Human)) | BDBM50299749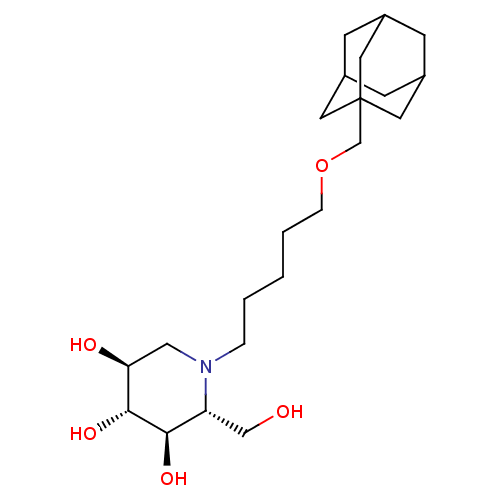 ((2R,3R,4R,5S)-1-[5-(Adamantan-1-ylmethoxy)-pentyl]...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis Curated by ChEMBL | Assay Description Inhibition of GCS assessed as amount of UDP-glucose consumed during enzyme-catalyzed reaction | Bioorg Med Chem Lett 21: 6773-7 (2011) Article DOI: 10.1016/j.bmcl.2011.09.037 BindingDB Entry DOI: 10.7270/Q2833SG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ceramide glucosyltransferase (Homo sapiens (Human)) | BDBM50299749 ((2R,3R,4R,5S)-1-[5-(Adamantan-1-ylmethoxy)-pentyl]...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis Curated by ChEMBL | Assay Description Inhibition of GCS assessed as amount of UDP glucose after 3 hrs by Fluorometry analysis | J Med Chem 55: 4322-35 (2012) Article DOI: 10.1021/jm300122u BindingDB Entry DOI: 10.7270/Q2MG7QMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ceramide glucosyltransferase (Homo sapiens (Human)) | BDBM50299749 ((2R,3R,4R,5S)-1-[5-(Adamantan-1-ylmethoxy)-pentyl]...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Inhibition of GCS by cell-based assay | J Med Chem 53: 689-98 (2010) Article DOI: 10.1021/jm901281m BindingDB Entry DOI: 10.7270/Q2M908TC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ceramide glucosyltransferase (Homo sapiens (Human)) | BDBM50299749 ((2R,3R,4R,5S)-1-[5-(Adamantan-1-ylmethoxy)-pentyl]...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Inhibition of glucosylceramide synthase (unknown origin) assessed as catabolism of NBD-glucosylceramide | J Med Chem 57: 9096-104 (2014) Article DOI: 10.1021/jm501181z BindingDB Entry DOI: 10.7270/Q20003PT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ceramide glucosyltransferase (Mus musculus) | BDBM50299749 ((2R,3R,4R,5S)-1-[5-(Adamantan-1-ylmethoxy)-pentyl]...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of glucosylceramide synthase in mouse RAW cells preincubated with compound for 15 mins by in-situ enzyme inhibition assay | ACS Med Chem Lett 2: 119-123 (2011) Article DOI: 10.1021/ml100192b BindingDB Entry DOI: 10.7270/Q21C1X5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ceramide glucosyltransferase (Mus musculus) | BDBM50299749 ((2R,3R,4R,5S)-1-[5-(Adamantan-1-ylmethoxy)-pentyl]...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of glucosylceramide synthase in mouse RAW cells preincubated for 15 mins followed by substrate addition and measured after 1 hr by fluores... | ACS Med Chem Lett 2: 519-522 (2011) Article DOI: 10.1021/ml200050s BindingDB Entry DOI: 10.7270/Q28916V8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ceramide glucosyltransferase (Homo sapiens (Human)) | BDBM50299749 ((2R,3R,4R,5S)-1-[5-(Adamantan-1-ylmethoxy)-pentyl]...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis Curated by ChEMBL | Assay Description Inhibition of GCS in human A549 cells assessed as decrease in GM1 synthesis after 72 hrs by Fluorescence assay | J Med Chem 55: 4322-35 (2012) Article DOI: 10.1021/jm300122u BindingDB Entry DOI: 10.7270/Q2MG7QMF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ceramide glucosyltransferase (Homo sapiens (Human)) | BDBM50299749 ((2R,3R,4R,5S)-1-[5-(Adamantan-1-ylmethoxy)-pentyl]...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Exelixis Curated by ChEMBL | Assay Description Inhibition of GCS activity in human A549 cells assessed as amount of GM1 on the cell membrane after 72 hrs by FL-CTB-based fluorescent microscopy | Bioorg Med Chem Lett 21: 6773-7 (2011) Article DOI: 10.1016/j.bmcl.2011.09.037 BindingDB Entry DOI: 10.7270/Q2833SG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||