

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Equus caballus (Horse)) | BDBM13551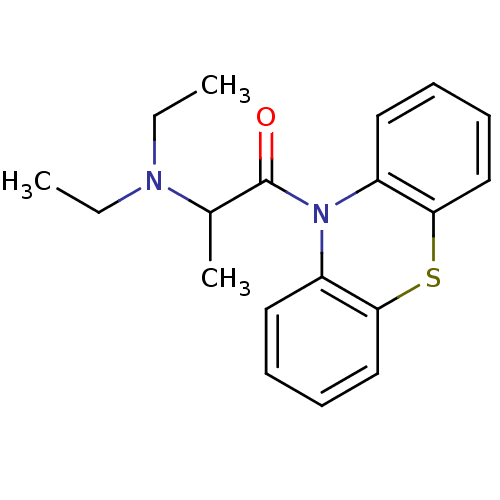 (10-[2-(Diethylamino)-1-oxopropyl]-10H-phenothiazin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Genova Curated by ChEMBL | Assay Description Inhibition of Equine serum BChE using butyrylthiocoline iodide as a substrate after 20 mins by Ellman's assay | Eur J Med Chem 46: 2170-84 (2011) Article DOI: 10.1016/j.ejmech.2011.02.071 BindingDB Entry DOI: 10.7270/Q2BK1CPG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM13551 (10-[2-(Diethylamino)-1-oxopropyl]-10H-phenothiazin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universitat Bonn | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changed at 412 nm and was monitored with spectr... | J Med Chem 49: 7540-4 (2006) Article DOI: 10.1021/jm060742o BindingDB Entry DOI: 10.7270/Q21Z42NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||