

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Homo sapiens (Human)) | BDBM50379274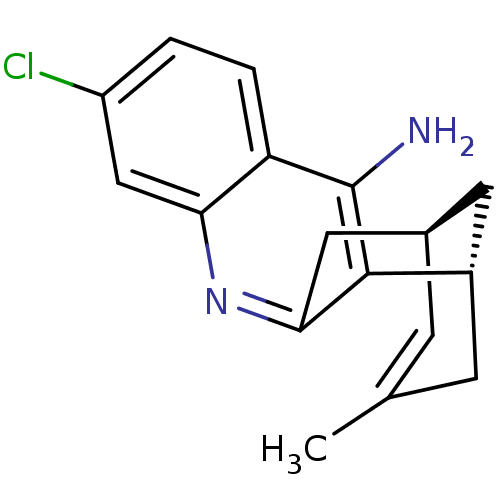 (CHEMBL2011499 | US9238626, (+)-Huprine Y HCl) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 170 | n/a | n/a | n/a | n/a | 8.0 | 25 |
UNIVERSITAT DE BARCELONA; PONTIFICIA UNIVERSIDAD CATÓLICA DE CHILE US Patent | Assay Description BChE inhibitory activity determinations were carried out similarly by the method of Ellman et al., using 0.02 unit/mL of human serum BChE and 300 u... | US Patent US9238626 (2016) BindingDB Entry DOI: 10.7270/Q2T152FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50379274 (CHEMBL2011499 | US9238626, (+)-Huprine Y HCl) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02150 BindingDB Entry DOI: 10.7270/Q2F76HMM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50379274 (CHEMBL2011499 | US9238626, (+)-Huprine Y HCl) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins by Ellman's method | J Med Chem 57: 2549-67 (2014) Article DOI: 10.1021/jm401824w BindingDB Entry DOI: 10.7270/Q2FX7BZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||