

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50088503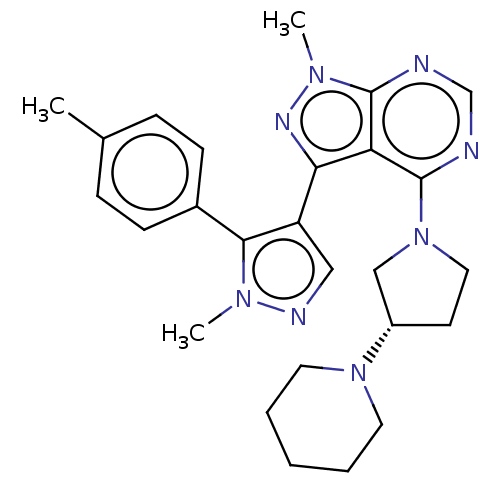 (CHEMBL3527048) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of testosterone 6beta-hydroxylase activity of human recombinant CYP3A5 in presence of human P450 oxidoreductase and b5 assessed as decreas... | Drug Metab Dispos 40: 1686-97 (2012) Article DOI: 10.1124/dmd.112.045302 BindingDB Entry DOI: 10.7270/Q2GT5PW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50088503 (CHEMBL3527048) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of midazolam 1'-hydroxylase activity of human recombinant CYP3A5 harboring human P450 oxidoreductase and b5 assessed as decrease in enzyme... | Drug Metab Dispos 40: 1686-97 (2012) Article DOI: 10.1124/dmd.112.045302 BindingDB Entry DOI: 10.7270/Q2GT5PW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50088503 (CHEMBL3527048) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Reversible inhibition of midazolam 1'-hydroxylase activity of human recombinant CYP3A5 harboring human P450 oxidoreductase and b5 | Drug Metab Dispos 40: 1686-97 (2012) Article DOI: 10.1124/dmd.112.045302 BindingDB Entry DOI: 10.7270/Q2GT5PW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A5 (Homo sapiens (Human)) | BDBM50088503 (CHEMBL3527048) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Reversible inhibition of testosterone 6beta-hydroxylase activity of human recombinant CYP3A5 in presence of human P450 oxidoreductase and b5 | Drug Metab Dispos 40: 1686-97 (2012) Article DOI: 10.1124/dmd.112.045302 BindingDB Entry DOI: 10.7270/Q2GT5PW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||