

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(1A) dopamine receptor (RAT) | BDBM50004918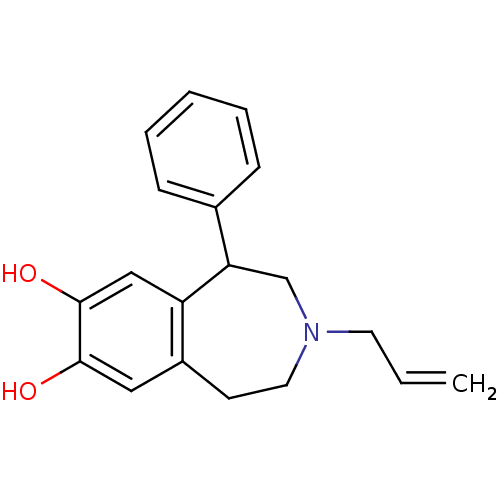 ((+/-)-APD3-Allyl-1-phenyl-2,3,4,5-tetrahydro-1H-be...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 10.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by PDSP Ki Database | Eur J Pharmacol 474: 137-40 (2003) Article DOI: 10.1016/s0014-2999(03)02008-9 BindingDB Entry DOI: 10.7270/Q2DN43MQ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50004918 ((+/-)-APD3-Allyl-1-phenyl-2,3,4,5-tetrahydro-1H-be...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description The compound was evaluated for the dissociation constant for inhibiting the binding of [3H]-SCH- 23390 at Dopamine receptor D1 | J Med Chem 34: 3366-71 (1992) BindingDB Entry DOI: 10.7270/Q26H4J17 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50004918 ((+/-)-APD3-Allyl-1-phenyl-2,3,4,5-tetrahydro-1H-be...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by PDSP Ki Database | NIDA Res Monogr 178: 440-66 (1998) BindingDB Entry DOI: 10.7270/Q23J3BH2 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50004918 ((+/-)-APD3-Allyl-1-phenyl-2,3,4,5-tetrahydro-1H-be...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Research Biochemicals Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-SCH- 23390 (0.3 nM) from dopamine receptor D1 in crude membrane fraction of rat brain corpus striatum | J Med Chem 35: 1466-71 (1992) BindingDB Entry DOI: 10.7270/Q26H4GCW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50004918 ((+/-)-APD3-Allyl-1-phenyl-2,3,4,5-tetrahydro-1H-be...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Agonist activity at human dopamine D1 receptor expressed in CHOK1 cells assessed as assessed as increase in beta-arrestin-2 recruitment after 60 mins... | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50004918 ((+/-)-APD3-Allyl-1-phenyl-2,3,4,5-tetrahydro-1H-be...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
ShanghaiTech University Curated by ChEMBL | Assay Description Agonist activity at human dopamine D1 receptor expressed in CHOK1 cells assessed as reversal of Ro 20-1724 mediated decrease in cAMP accumulation aft... | J Med Chem 61: 9841-9878 (2018) Article DOI: 10.1021/acs.jmedchem.8b00435 BindingDB Entry DOI: 10.7270/Q2F76GX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50004918 ((+/-)-APD3-Allyl-1-phenyl-2,3,4,5-tetrahydro-1H-be...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Dopamine agonist (Dopamine receptor D1) activity was measured as increase in cAMP formation relative to maximum increase in dopamine-sensitive adenyl... | J Med Chem 30: 35-40 (1987) BindingDB Entry DOI: 10.7270/Q2X92BV6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||