

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50048342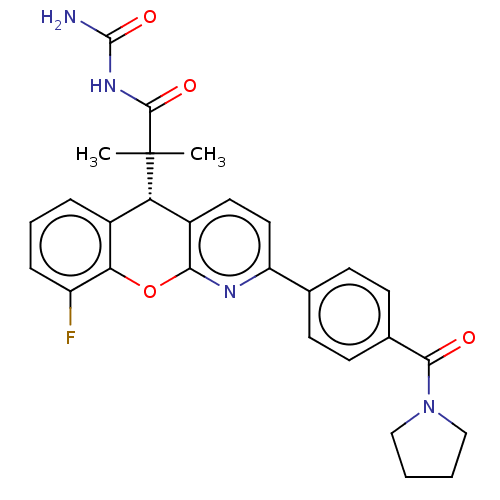 (CHEMBL3315064 | US9593113, Example 30) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity to glucocorticoid receptor (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 24: 3268-73 (2014) Article DOI: 10.1016/j.bmcl.2014.06.010 BindingDB Entry DOI: 10.7270/Q21N82SN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50048342 (CHEMBL3315064 | US9593113, Example 30) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company US Patent | Assay Description Binding Assay (I):In order to assess the affinity of test compounds for the human glucocorticoid receptor, a commercially available kit was used (Glu... | US Patent US9593113 (2017) BindingDB Entry DOI: 10.7270/Q2833V3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50048342 (CHEMBL3315064 | US9593113, Example 30) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Agonist activity at glucocorticoid receptor in human A549 cells assessed as E-selectin transrepression by luciferase reporter gene assay | Bioorg Med Chem Lett 24: 3268-73 (2014) Article DOI: 10.1016/j.bmcl.2014.06.010 BindingDB Entry DOI: 10.7270/Q21N82SN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50048342 (CHEMBL3315064 | US9593113, Example 30) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Agonist activity at glucocorticoid receptor in human A549 cells assessed as AP-1 transrepression by luciferase reporter gene assay | Bioorg Med Chem Lett 24: 3268-73 (2014) Article DOI: 10.1016/j.bmcl.2014.06.010 BindingDB Entry DOI: 10.7270/Q21N82SN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50048342 (CHEMBL3315064 | US9593113, Example 30) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Agonist activity at glucocorticoid receptor in human HeLa cells assessed as NP-1 transactivation by luciferase reporter gene assay | Bioorg Med Chem Lett 24: 3268-73 (2014) Article DOI: 10.1016/j.bmcl.2014.06.010 BindingDB Entry DOI: 10.7270/Q21N82SN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||