

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50158601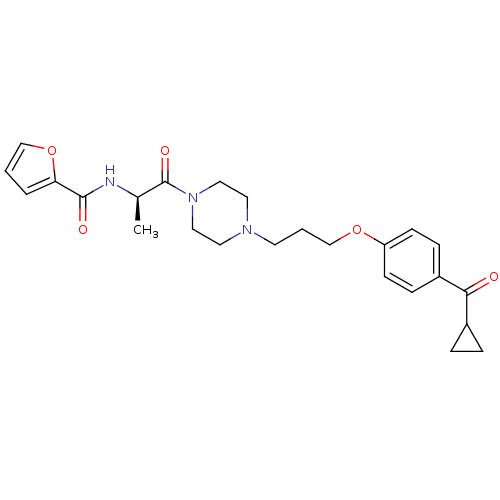 (A-317920 | CHEMBL361355 | Furan-2-carboxylic acid ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity to rat cortical histamine H3 receptor | J Med Chem 54: 26-53 (2011) Article DOI: 10.1021/jm100064d BindingDB Entry DOI: 10.7270/Q2VQ33RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50158601 (A-317920 | CHEMBL361355 | Furan-2-carboxylic acid ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity was determined as displacement of [3H]N-R-methylhistamine from C6 cell membranes expressing rat histamine H3 receptor | J Med Chem 48: 38-55 (2005) Article DOI: 10.1021/jm040118g BindingDB Entry DOI: 10.7270/Q2571CST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50158601 (A-317920 | CHEMBL361355 | Furan-2-carboxylic acid ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by PDSP Ki Database | J Pharmacol Exp Ther 305: 887-96 (2003) Article DOI: 10.1124/jpet.102.047183 BindingDB Entry DOI: 10.7270/Q22J69FK | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50158601 (A-317920 | CHEMBL361355 | Furan-2-carboxylic acid ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity was determined as displacement of [3H]N-R-methylhistamine from C6 cell membranes expressing human histamine H3 receptor | J Med Chem 48: 38-55 (2005) Article DOI: 10.1021/jm040118g BindingDB Entry DOI: 10.7270/Q2571CST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50158601 (A-317920 | CHEMBL361355 | Furan-2-carboxylic acid ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 93.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by PDSP Ki Database | J Pharmacol Exp Ther 305: 887-96 (2003) Article DOI: 10.1124/jpet.102.047183 BindingDB Entry DOI: 10.7270/Q22J69FK | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50158601 (A-317920 | CHEMBL361355 | Furan-2-carboxylic acid ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity to human histamine H3 receptor | J Med Chem 54: 26-53 (2011) Article DOI: 10.1021/jm100064d BindingDB Entry DOI: 10.7270/Q2VQ33RV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50158601 (A-317920 | CHEMBL361355 | Furan-2-carboxylic acid ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by PDSP Ki Database | J Pharmacol Exp Ther 305: 887-96 (2003) Article DOI: 10.1124/jpet.102.047183 BindingDB Entry DOI: 10.7270/Q22J69FK | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||