

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM26066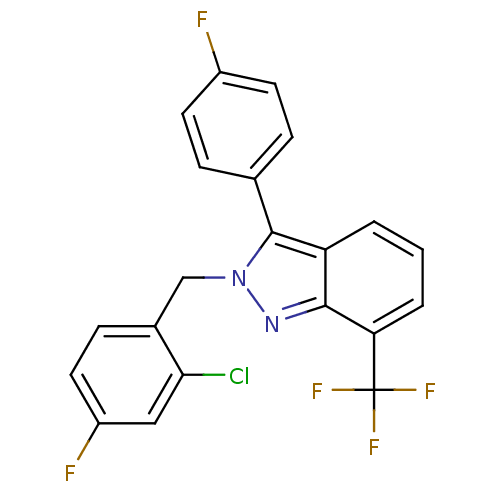 (2-[(2-chloro-4-fluorophenyl)methyl]-3-(4-fluorophe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Binding affinity to recombinant human LXRbeta-LBD expressed in Escherichia coli BL21 (DE3) assessed as inhibitory constant incubated for 30 mins by f... | Eur J Med Chem 178: 458-467 (2019) Article DOI: 10.1016/j.ejmech.2019.06.011 BindingDB Entry DOI: 10.7270/Q2BC42WH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM26066 (2-[(2-chloro-4-fluorophenyl)methyl]-3-(4-fluorophe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of hyodeoxycholicacid-based fluorescent tracer from recombinant human LXRbeta LBD (215 to 461 residues) expressed in Escherichia coli BL... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112240 BindingDB Entry DOI: 10.7270/Q2MG7T4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM26066 (2-[(2-chloro-4-fluorophenyl)methyl]-3-(4-fluorophe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | n/a | 24 | n/a | n/a | n/a | n/a |
The Rockefeller University US Patent | Assay Description As used herein, reference to the activity of an LXR agonist at LXRα and LXRβ refer to the activity as measured using the ligand sensing ass... | US Patent US10543183 (2020) BindingDB Entry DOI: 10.7270/Q2JW8H8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM26066 (2-[(2-chloro-4-fluorophenyl)methyl]-3-(4-fluorophe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | n/a | 24 | n/a | n/a | n/a | n/a |
The Rockefeller University US Patent | Assay Description As used herein, reference to the activity of an LXR agonist at LXRα and LXRβ refer to the activity as measured using the ligand sensing ass... | US Patent US10945978 (2021) BindingDB Entry DOI: 10.7270/Q2959MPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM26066 (2-[(2-chloro-4-fluorophenyl)methyl]-3-(4-fluorophe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | n/a | 24 | n/a | n/a | n/a | n/a |
Rgenix, Inc. US Patent | Assay Description TBD | US Patent US10669296 (2020) BindingDB Entry DOI: 10.7270/Q2V127W1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM26066 (2-[(2-chloro-4-fluorophenyl)methyl]-3-(4-fluorophe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 900 | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt Curated by ChEMBL | Assay Description Partial agonist activity at recombinant human GAL4-DBD-fused LXRbeta-LBD expressed in HEK293T cells measured after 12 to 14 hrs by dual-glo luciferas... | Bioorg Med Chem Lett 27: 1193-1198 (2017) Article DOI: 10.1016/j.bmcl.2017.01.066 BindingDB Entry DOI: 10.7270/Q2668GNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM26066 (2-[(2-chloro-4-fluorophenyl)methyl]-3-(4-fluorophe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.67E+3 | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human LXRbeta ligand binding domain expressed in human HuH7 cells co-transfected with Gal4-DBD by transactivation assay | Bioorg Med Chem Lett 20: 689-93 (2010) Article DOI: 10.1016/j.bmcl.2009.11.062 BindingDB Entry DOI: 10.7270/Q2W66KV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||