| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Oxysterols receptor LXR-beta |
|---|
| Ligand | BDBM26066 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_604020 (CHEMBL1049548) |
|---|
| EC50 | 3670±n/a nM |
|---|
| Citation |  Hu, B; Bernotas, R; Unwalla, R; Collini, M; Quinet, E; Feingold, I; Goos-Nilsson, A; Wilhelmsson, A; Nambi, P; Evans, M; Wrobel, J Quinoline-3-carboxamide containing sulfones as liver X receptor (LXR) agonists with binding selectivity for LXRbeta and low blood-brain penetration. Bioorg Med Chem Lett20:689-93 (2010) [PubMed] Article Hu, B; Bernotas, R; Unwalla, R; Collini, M; Quinet, E; Feingold, I; Goos-Nilsson, A; Wilhelmsson, A; Nambi, P; Evans, M; Wrobel, J Quinoline-3-carboxamide containing sulfones as liver X receptor (LXR) agonists with binding selectivity for LXRbeta and low blood-brain penetration. Bioorg Med Chem Lett20:689-93 (2010) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Oxysterols receptor LXR-beta |
|---|
| Name: | Oxysterols receptor LXR-beta |
|---|
| Synonyms: | LXRB | Liver X receptor beta (NR1H2) | Liver X, LXR beta | NER | NR1H2 | NR1H2_HUMAN | Nuclear receptor NER | UNR | Ubiquitously-expressed nuclear receptor |
|---|
| Type: | Enzyme Catalytic Domain |
|---|
| Mol. Mass.: | 50978.79 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P55055 |
|---|
| Residue: | 460 |
|---|
| Sequence: | MSSPTTSSLDTPLPGNGPPQPGAPSSSPTVKEEGPEPWPGGPDPDVPGTDEASSACSTDW
VIPDPEEEPERKRKKGPAPKMLGHELCRVCGDKASGFHYNVLSCEGCKGFFRRSVVRGGA
RRYACRGGGTCQMDAFMRRKCQQCRLRKCKEAGMREQCVLSEEQIRKKKIRKQQQESQSQ
SQSPVGPQGSSSSASGPGASPGGSEAGSQGSGEGEGVQLTAAQELMIQQLVAAQLQCNKR
SFSDQPKVTPWPLGADPQSRDARQQRFAHFTELAIISVQEIVDFAKQVPGFLQLGREDQI
ALLKASTIEIMLLETARRYNHETECITFLKDFTYSKDDFHRAGLQVEFINPIFEFSRAMR
RLGLDDAEYALLIAINIFSADRPNVQEPGRVEALQQPYVEALLSYTRIKRPQDQLRFPRM
LMKLVSLRTLSSVHSEQVFALRLQDKKLPPLLSEIWDVHE
|
|
|
|---|
| BDBM26066 |
|---|
| n/a |
|---|
| Name | BDBM26066 |
|---|
| Synonyms: | 2-[(2-chloro-4-fluorophenyl)methyl]-3-(4-fluorophenyl)-7-(trifluoromethyl)-2H-indazole | JMC517161 Compound 12 | US10543183, Compound LXR-623 | US10669296, Compound LXR-623 | US10945978, Compound 3 | WAY-252623 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C21H12ClF5N2 |
|---|
| Mol. Mass. | 422.778 |
|---|
| SMILES | Fc1ccc(cc1)-c1n(Cc2ccc(F)cc2Cl)nc2c(cccc12)C(F)(F)F |
|---|
| Structure | 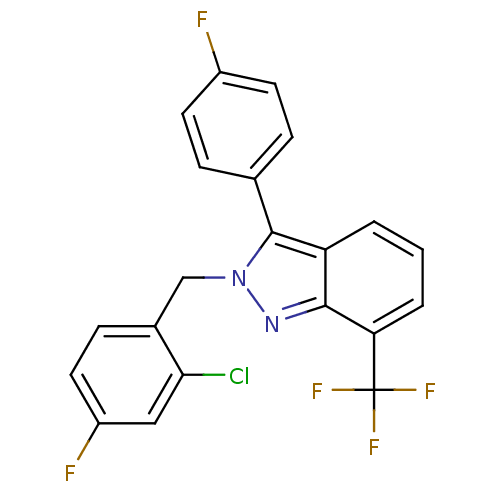
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Hu, B; Bernotas, R; Unwalla, R; Collini, M; Quinet, E; Feingold, I; Goos-Nilsson, A; Wilhelmsson, A; Nambi, P; Evans, M; Wrobel, J Quinoline-3-carboxamide containing sulfones as liver X receptor (LXR) agonists with binding selectivity for LXRbeta and low blood-brain penetration. Bioorg Med Chem Lett20:689-93 (2010) [PubMed] Article
Hu, B; Bernotas, R; Unwalla, R; Collini, M; Quinet, E; Feingold, I; Goos-Nilsson, A; Wilhelmsson, A; Nambi, P; Evans, M; Wrobel, J Quinoline-3-carboxamide containing sulfones as liver X receptor (LXR) agonists with binding selectivity for LXRbeta and low blood-brain penetration. Bioorg Med Chem Lett20:689-93 (2010) [PubMed] Article