

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466897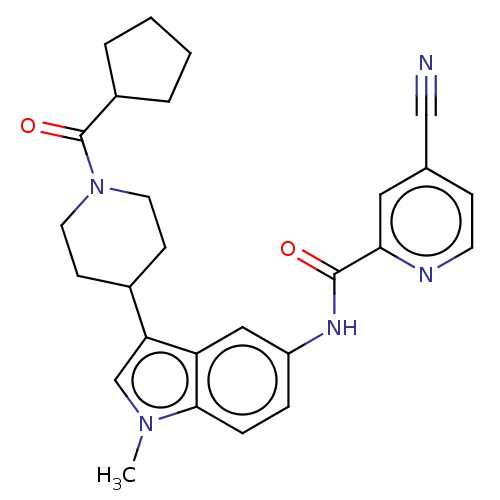 (CHEMBL4287715) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466891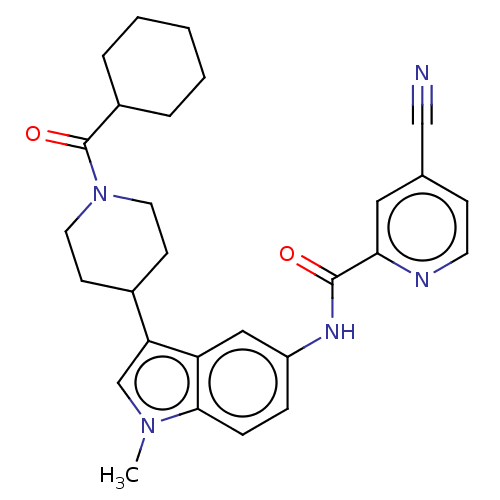 (CHEMBL4281109) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466893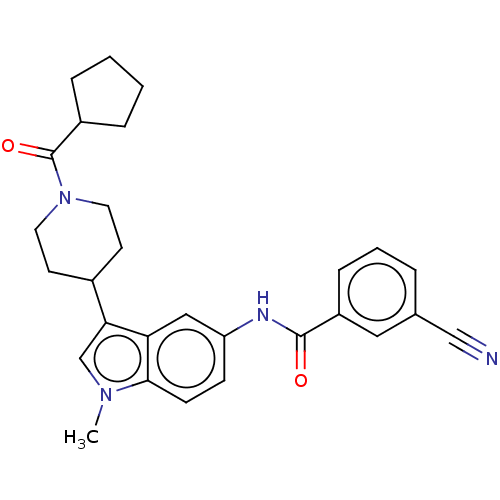 (CHEMBL4291727) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466892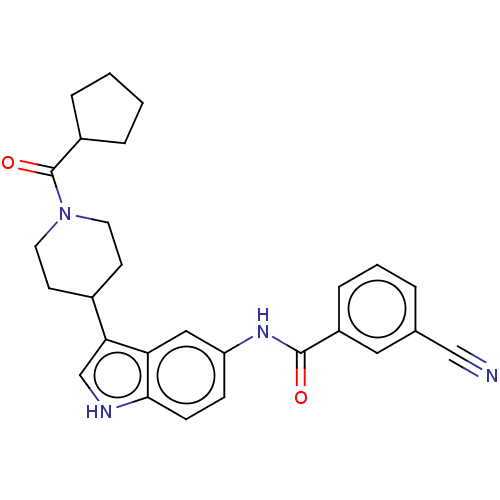 (CHEMBL4283871) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466913 (CHEMBL4289304) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50306072 (2-isobutyl-1-(3-(3-(methylsulfonyl)phenoxy)phenyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRalpha LBD | Bioorg Med Chem Lett 20: 526-30 (2010) Article DOI: 10.1016/j.bmcl.2009.11.099 BindingDB Entry DOI: 10.7270/Q2HT2PDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50378590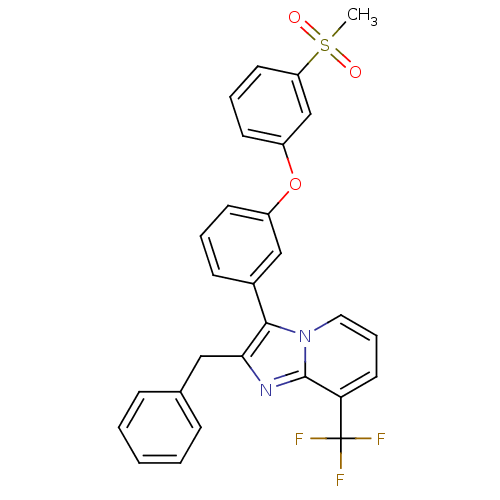 (CHEMBL611735) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRalpha LBD | Bioorg Med Chem Lett 20: 521-5 (2010) Article DOI: 10.1016/j.bmcl.2009.11.098 BindingDB Entry DOI: 10.7270/Q27D2W3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50305075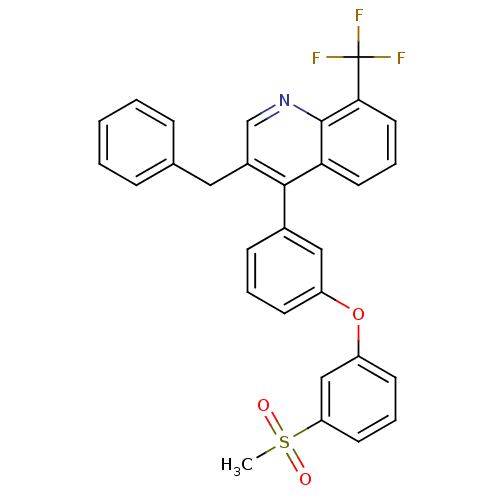 (3-benzyl-4-(3-(3-(methylsulfonyl)phenoxy)phenyl)-8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRalpha-LBD | Bioorg Med Chem Lett 20: 209-12 (2010) Article DOI: 10.1016/j.bmcl.2009.10.132 BindingDB Entry DOI: 10.7270/Q2KW5G4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50305077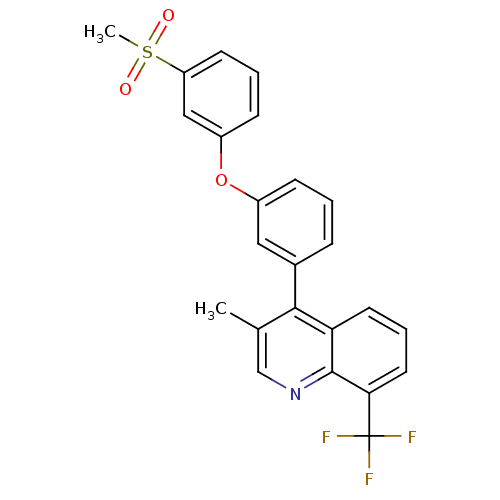 (3-methyl-4-(3-(3-(methylsulfonyl)phenoxy)phenyl)-8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRalpha-LBD | Bioorg Med Chem Lett 20: 209-12 (2010) Article DOI: 10.1016/j.bmcl.2009.10.132 BindingDB Entry DOI: 10.7270/Q2KW5G4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50306070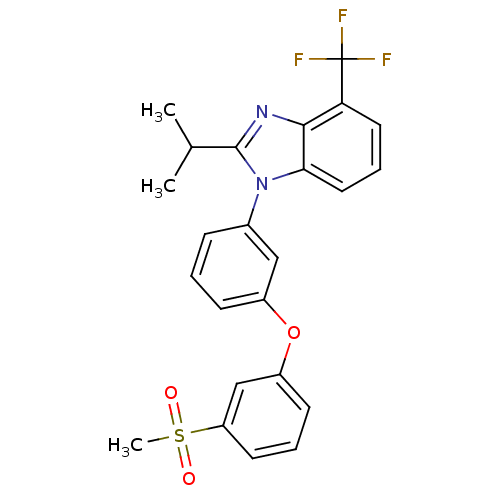 (2-isopropyl-1-(3-(3-(methylsulfonyl)phenoxy)phenyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRalpha LBD | Bioorg Med Chem Lett 20: 526-30 (2010) Article DOI: 10.1016/j.bmcl.2009.11.099 BindingDB Entry DOI: 10.7270/Q2HT2PDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50305077 (3-methyl-4-(3-(3-(methylsulfonyl)phenoxy)phenyl)-8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRalpha LBD | Bioorg Med Chem Lett 20: 526-30 (2010) Article DOI: 10.1016/j.bmcl.2009.11.099 BindingDB Entry DOI: 10.7270/Q2HT2PDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50305077 (3-methyl-4-(3-(3-(methylsulfonyl)phenoxy)phenyl)-8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRalpha LBD | Bioorg Med Chem Lett 20: 521-5 (2010) Article DOI: 10.1016/j.bmcl.2009.11.098 BindingDB Entry DOI: 10.7270/Q27D2W3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50305077 (3-methyl-4-(3-(3-(methylsulfonyl)phenoxy)phenyl)-8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]TO901317 from human recombinant LXRbeta expressed in Escherichia coli by flashplate method | Bioorg Med Chem Lett 20: 689-93 (2010) Article DOI: 10.1016/j.bmcl.2009.11.062 BindingDB Entry DOI: 10.7270/Q2W66KV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50306073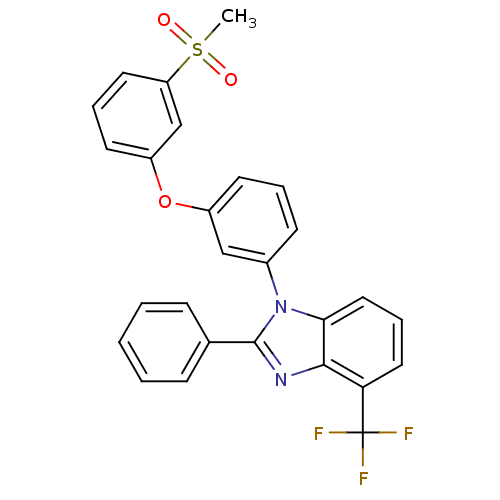 (1-(3-(3-(methylsulfonyl)phenoxy)phenyl)-2-phenyl-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRalpha LBD | Bioorg Med Chem Lett 20: 526-30 (2010) Article DOI: 10.1016/j.bmcl.2009.11.099 BindingDB Entry DOI: 10.7270/Q2HT2PDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50305072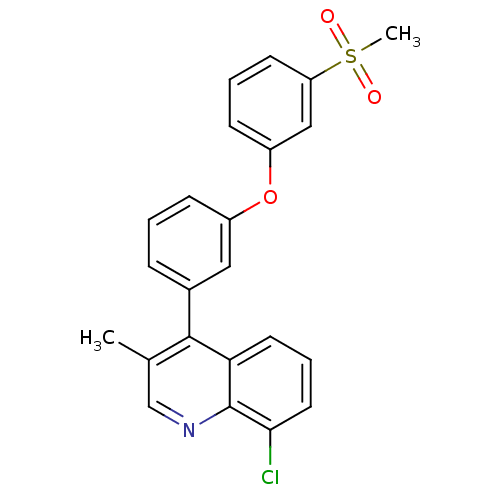 (8-chloro-3-methyl-4-(3-(3-(methylsulfonyl)phenoxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRalpha-LBD | Bioorg Med Chem Lett 20: 209-12 (2010) Article DOI: 10.1016/j.bmcl.2009.10.132 BindingDB Entry DOI: 10.7270/Q2KW5G4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50306074 (2-(4-fluorobenzyl)-1-(3-(3-(methylsulfonyl)phenoxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRalpha LBD | Bioorg Med Chem Lett 20: 526-30 (2010) Article DOI: 10.1016/j.bmcl.2009.11.099 BindingDB Entry DOI: 10.7270/Q2HT2PDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50305073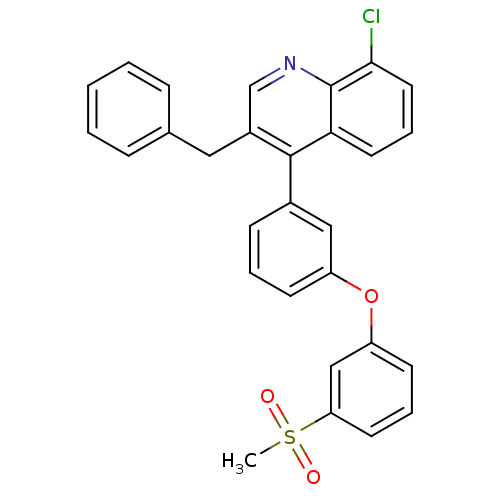 (3-benzyl-8-chloro-4-(3-(3-(methylsulfonyl)phenoxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRalpha-LBD | Bioorg Med Chem Lett 20: 209-12 (2010) Article DOI: 10.1016/j.bmcl.2009.10.132 BindingDB Entry DOI: 10.7270/Q2KW5G4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50306068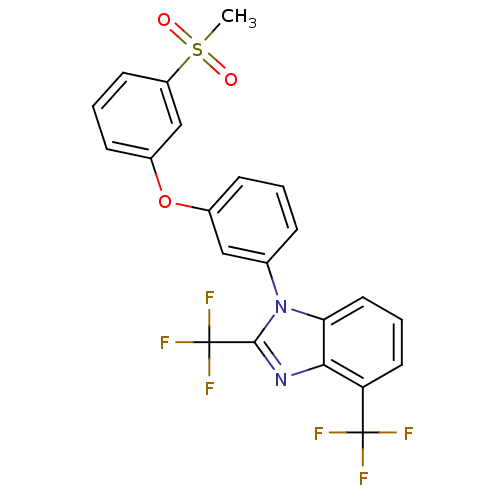 (1-(3-(3-(methylsulfonyl)phenoxy)phenyl)-2,4-bis(tr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRalpha LBD | Bioorg Med Chem Lett 20: 526-30 (2010) Article DOI: 10.1016/j.bmcl.2009.11.099 BindingDB Entry DOI: 10.7270/Q2HT2PDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM20015 (2-[4-({[3-(3-benzyl-8-chloroquinolin-4-yl)phenyl]a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | 23 | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50305064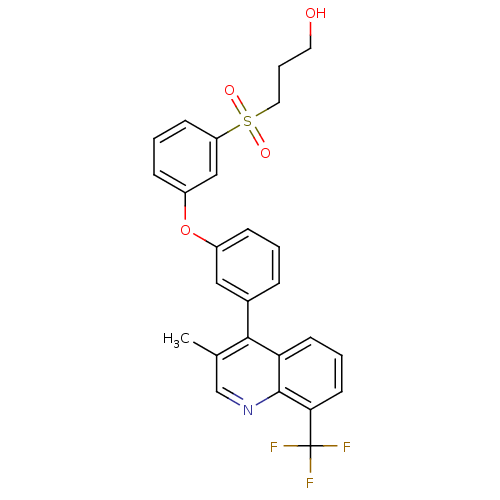 (3-(3-(3-(3-methyl-8-(trifluoromethyl)quinolin-4-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRalpha-LBD | Bioorg Med Chem Lett 20: 209-12 (2010) Article DOI: 10.1016/j.bmcl.2009.10.132 BindingDB Entry DOI: 10.7270/Q2KW5G4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM20024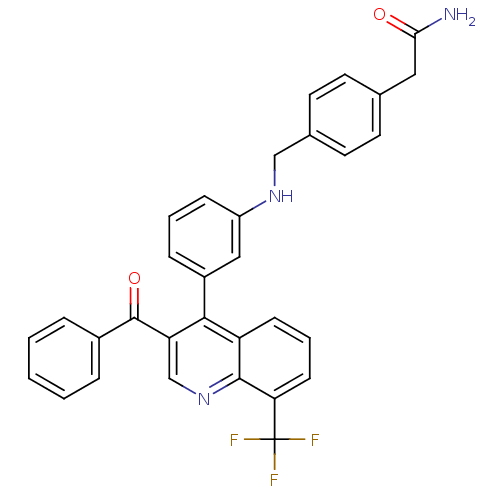 (2-{4-[({3-[3-benzoyl-8-(trifluoromethyl)quinolin-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | 316 | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50317735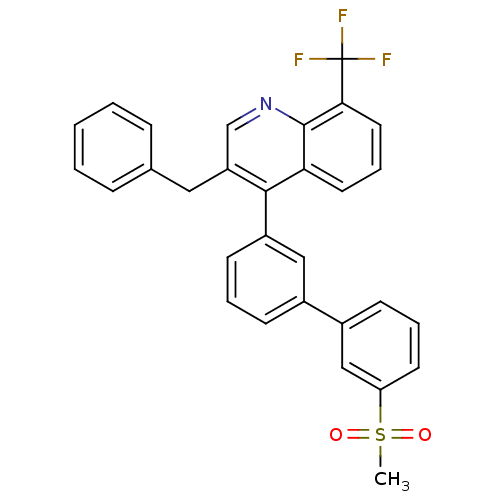 (3-benzyl-4-(3'-(methylsulfonyl)biphenyl-3-yl)-8-(t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from LXRbeta ligand binding domain | Bioorg Med Chem Lett 20: 2903-7 (2010) Article DOI: 10.1016/j.bmcl.2010.03.031 BindingDB Entry DOI: 10.7270/Q2TD9XJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50306075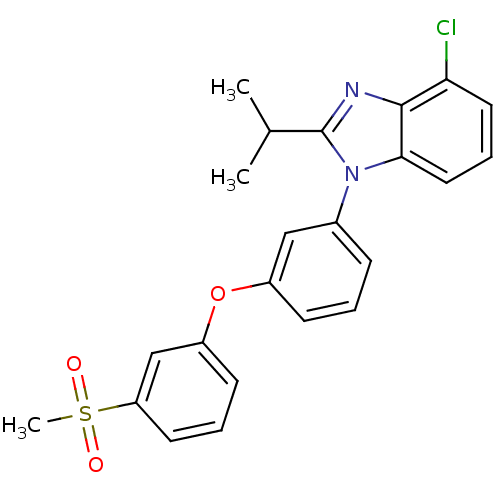 (4-chloro-2-isopropyl-1-(3-(3-(methylsulfonyl)pheno...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRalpha LBD | Bioorg Med Chem Lett 20: 526-30 (2010) Article DOI: 10.1016/j.bmcl.2009.11.099 BindingDB Entry DOI: 10.7270/Q2HT2PDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50305070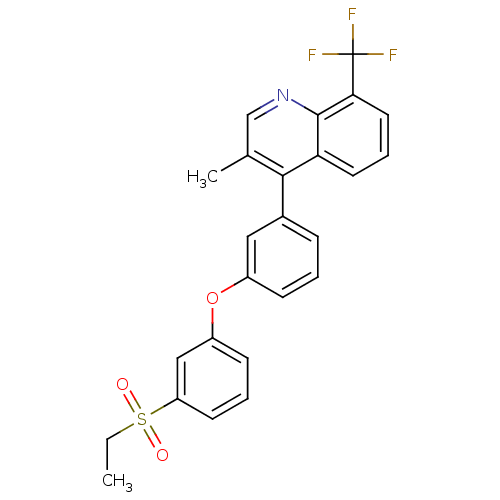 (4-(3-(3-(ethylsulfonyl)phenoxy)phenyl)-3-methyl-8-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRalpha-LBD | Bioorg Med Chem Lett 20: 209-12 (2010) Article DOI: 10.1016/j.bmcl.2009.10.132 BindingDB Entry DOI: 10.7270/Q2KW5G4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50317744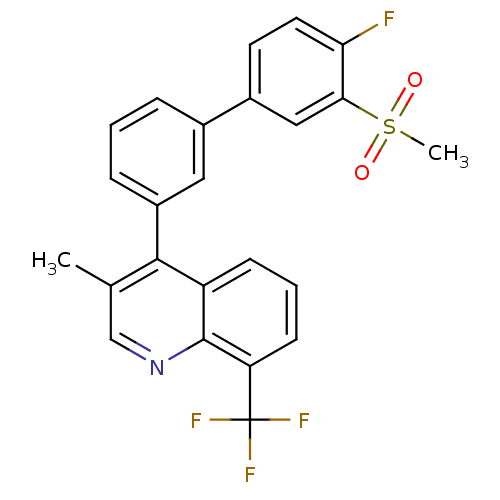 (4-(4'-fluoro-3'-(methylsulfonyl)biphenyl-3-yl)-3-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from LXRbeta ligand binding domain | Bioorg Med Chem Lett 20: 2903-7 (2010) Article DOI: 10.1016/j.bmcl.2010.03.031 BindingDB Entry DOI: 10.7270/Q2TD9XJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50306069 (2-ethyl-1-(3-(3-(methylsulfonyl)phenoxy)phenyl)-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRalpha LBD | Bioorg Med Chem Lett 20: 526-30 (2010) Article DOI: 10.1016/j.bmcl.2009.11.099 BindingDB Entry DOI: 10.7270/Q2HT2PDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50317733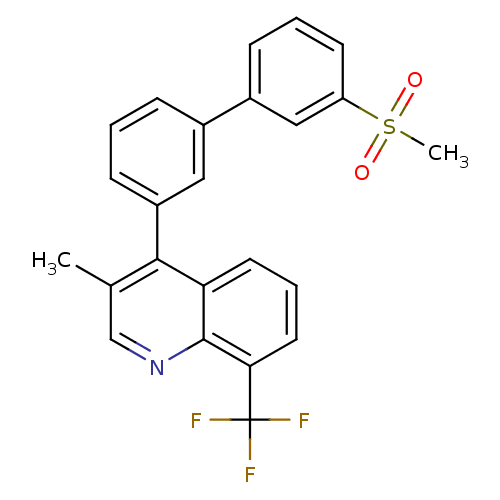 (3-methyl-4-(3'-(methylsulfonyl)biphenyl-3-yl)-8-(t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from LXRbeta ligand binding domain | Bioorg Med Chem Lett 20: 2903-7 (2010) Article DOI: 10.1016/j.bmcl.2010.03.031 BindingDB Entry DOI: 10.7270/Q2TD9XJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50305068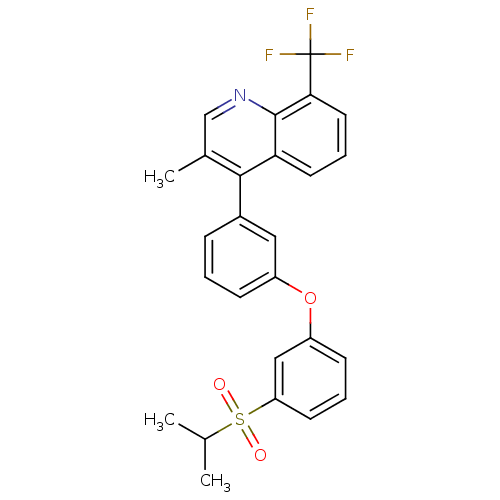 (4-(3-(3-(isopropylsulfonyl)phenoxy)phenyl)-3-methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRalpha-LBD | Bioorg Med Chem Lett 20: 209-12 (2010) Article DOI: 10.1016/j.bmcl.2009.10.132 BindingDB Entry DOI: 10.7270/Q2KW5G4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM20001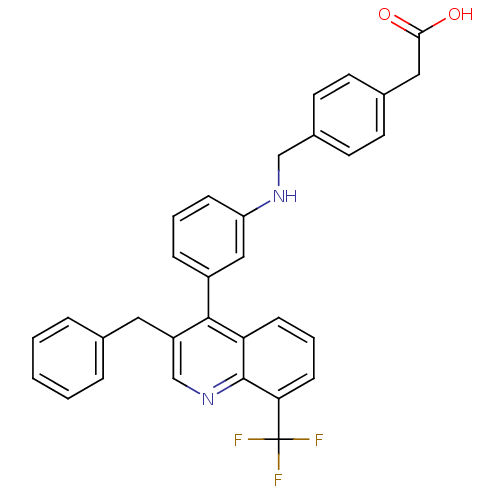 (2-{4-[({3-[3-benzyl-8-(trifluoromethyl)quinolin-4-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90 | n/a | 33 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | J Med Chem 49: 6151-4 (2006) Article DOI: 10.1021/jm0609566 BindingDB Entry DOI: 10.7270/Q20863KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM20001 (2-{4-[({3-[3-benzyl-8-(trifluoromethyl)quinolin-4-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90 | n/a | 33 | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM35107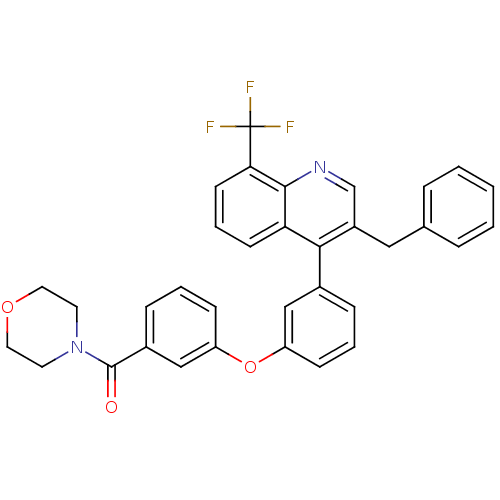 (biarylether amide quinoline, 4g) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | 138 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 17: 1663-70 (2009) Article DOI: 10.1016/j.bmc.2008.12.048 BindingDB Entry DOI: 10.7270/Q2QR4VGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50305060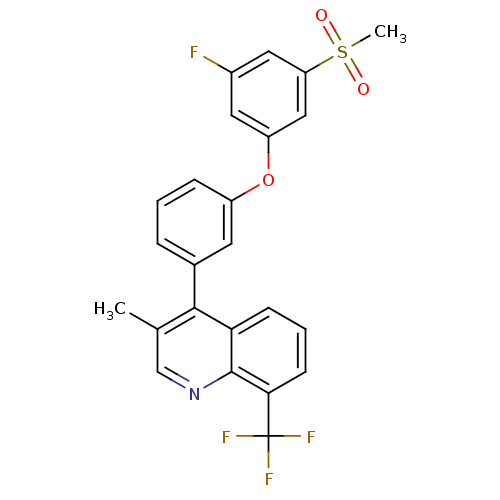 (4-(3-(3-fluoro-5-(methylsulfonyl)phenoxy)phenyl)-3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRalpha-LBD | Bioorg Med Chem Lett 20: 209-12 (2010) Article DOI: 10.1016/j.bmcl.2009.10.132 BindingDB Entry DOI: 10.7270/Q2KW5G4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50378593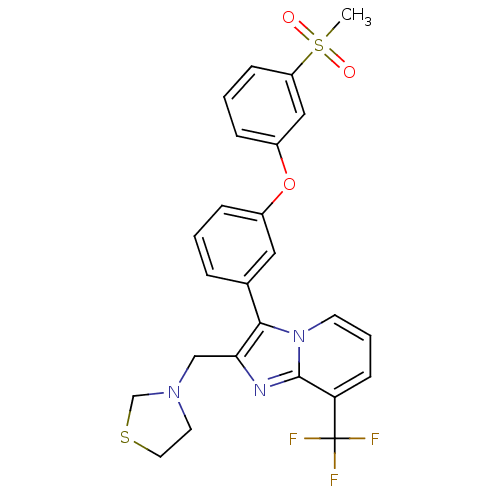 (CHEMBL612007) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRalpha LBD | Bioorg Med Chem Lett 20: 521-5 (2010) Article DOI: 10.1016/j.bmcl.2009.11.098 BindingDB Entry DOI: 10.7270/Q27D2W3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50227161 (2-(4-(3-(3-benzyl-8-(trifluoromethyl)quinolin-4-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity at human LXRbeta | Bioorg Med Chem Lett 18: 54-9 (2008) Article DOI: 10.1016/j.bmcl.2007.11.013 BindingDB Entry DOI: 10.7270/Q2N879JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50227146 (2-(4-(3-(3-benzyl-8-(trifluoromethyl)quinolin-4-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity at human LXRbeta | Bioorg Med Chem Lett 18: 54-9 (2008) Article DOI: 10.1016/j.bmcl.2007.11.013 BindingDB Entry DOI: 10.7270/Q2N879JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50227163 (2-(4-((3-(3-benzoyl-8-(trifluoromethyl)quinolin-5-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity at human LXRbeta | Bioorg Med Chem Lett 18: 54-9 (2008) Article DOI: 10.1016/j.bmcl.2007.11.013 BindingDB Entry DOI: 10.7270/Q2N879JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM20000 (2-(4-{3-[3-benzyl-8-(trifluoromethyl)quinolin-4-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity at human LXRbeta | Bioorg Med Chem Lett 18: 54-9 (2008) Article DOI: 10.1016/j.bmcl.2007.11.013 BindingDB Entry DOI: 10.7270/Q2N879JT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50227157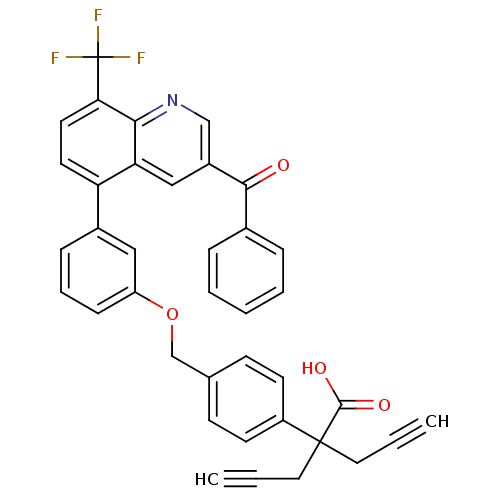 (2-(4-((3-(3-benzoyl-8-(trifluoromethyl)quinolin-5-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity at human LXRalpha | Bioorg Med Chem Lett 18: 54-9 (2008) Article DOI: 10.1016/j.bmcl.2007.11.013 BindingDB Entry DOI: 10.7270/Q2N879JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50305501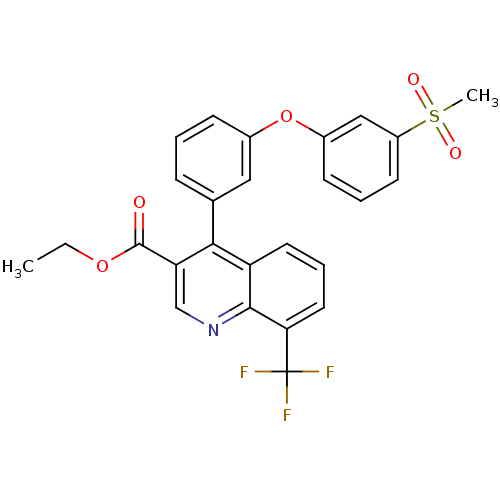 (CHEMBL590098 | ethyl 4-(3-(3-(methylsulfonyl)pheno...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]TO901317 from human recombinant LXRbeta expressed in Escherichia coli by flashplate method | Bioorg Med Chem Lett 20: 689-93 (2010) Article DOI: 10.1016/j.bmcl.2009.11.062 BindingDB Entry DOI: 10.7270/Q2W66KV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50305075 (3-benzyl-4-(3-(3-(methylsulfonyl)phenoxy)phenyl)-8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRbeta-LBD | Bioorg Med Chem Lett 20: 209-12 (2010) Article DOI: 10.1016/j.bmcl.2009.10.132 BindingDB Entry DOI: 10.7270/Q2KW5G4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50305077 (3-methyl-4-(3-(3-(methylsulfonyl)phenoxy)phenyl)-8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]TO901317 from human recombinant LXRalpha expressed in Escherichia coli by flashplate method | Bioorg Med Chem Lett 20: 689-93 (2010) Article DOI: 10.1016/j.bmcl.2009.11.062 BindingDB Entry DOI: 10.7270/Q2W66KV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM20000 (2-(4-{3-[3-benzyl-8-(trifluoromethyl)quinolin-4-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.10 | n/a | 93 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 17: 1663-70 (2009) Article DOI: 10.1016/j.bmc.2008.12.048 BindingDB Entry DOI: 10.7270/Q2QR4VGV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM35094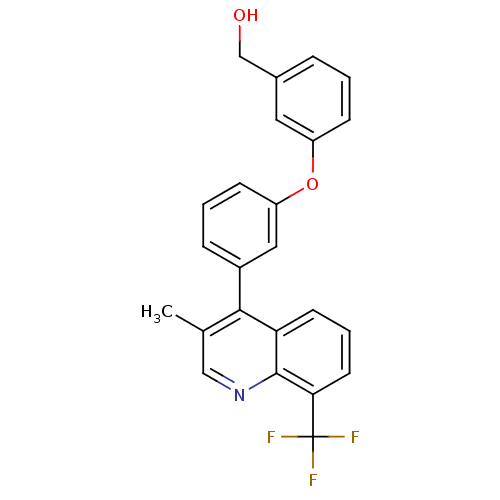 (biarylether alcohol quinoline, 5f) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | 233 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRbeta coated flash plates. Each concentration of ... | Bioorg Med Chem 17: 8086-92 (2009) Article DOI: 10.1016/j.bmc.2009.10.001 BindingDB Entry DOI: 10.7270/Q2VH5M54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM20000 (2-(4-{3-[3-benzyl-8-(trifluoromethyl)quinolin-4-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.10 | n/a | 71 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | J Med Chem 49: 6151-4 (2006) Article DOI: 10.1021/jm0609566 BindingDB Entry DOI: 10.7270/Q20863KH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM20000 (2-(4-{3-[3-benzyl-8-(trifluoromethyl)quinolin-4-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.10 | n/a | 71 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRbeta coated flash plates. Each concentration of ... | Bioorg Med Chem 17: 8086-92 (2009) Article DOI: 10.1016/j.bmc.2009.10.001 BindingDB Entry DOI: 10.7270/Q2VH5M54 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50305499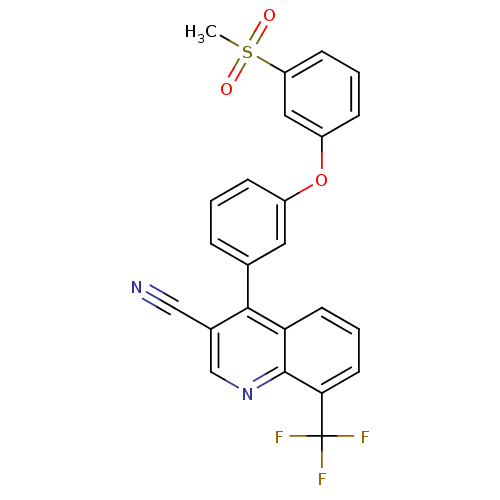 (4-(3-(3-(methylsulfonyl)phenoxy)phenyl)-8-(trifluo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]TO901317 from human recombinant LXRbeta expressed in Escherichia coli by flashplate method | Bioorg Med Chem Lett 20: 689-93 (2010) Article DOI: 10.1016/j.bmcl.2009.11.062 BindingDB Entry DOI: 10.7270/Q2W66KV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50317737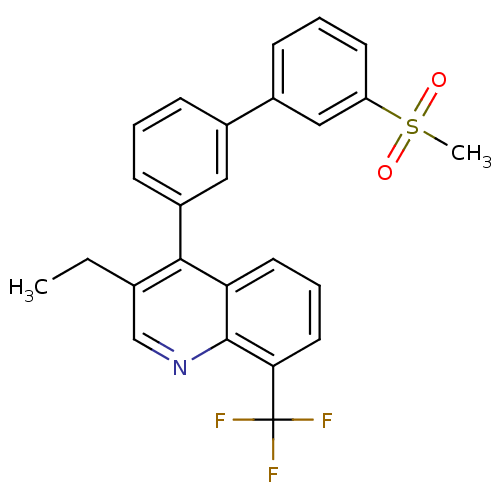 (3-ethyl-4-(3'-(methylsulfonyl)biphenyl-3-yl)-8-(tr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from LXRbeta ligand binding domain | Bioorg Med Chem Lett 20: 2903-7 (2010) Article DOI: 10.1016/j.bmcl.2010.03.031 BindingDB Entry DOI: 10.7270/Q2TD9XJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50306077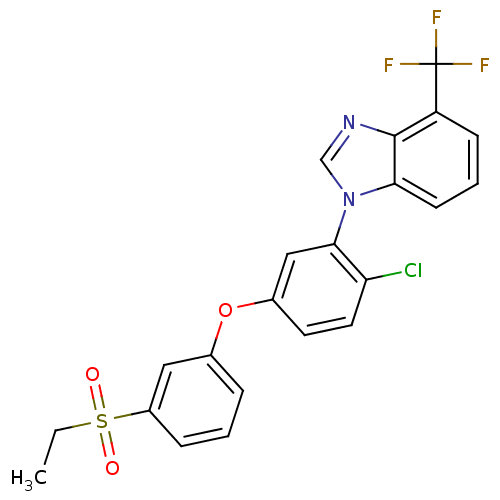 (1-(2-chloro-5-(3-(ethylsulfonyl)phenoxy)phenyl)-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRalpha LBD | Bioorg Med Chem Lett 20: 526-30 (2010) Article DOI: 10.1016/j.bmcl.2009.11.099 BindingDB Entry DOI: 10.7270/Q2HT2PDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM20004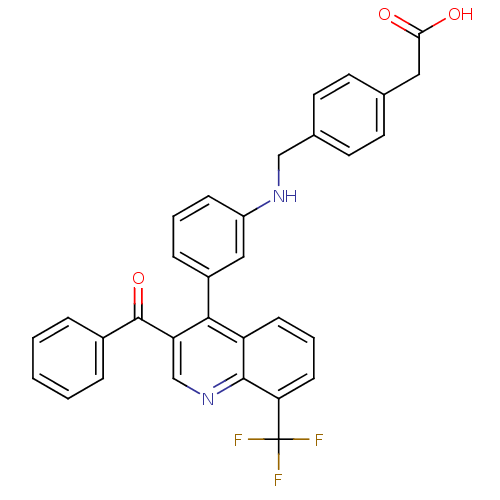 (2-{4-[({3-[3-benzoyl-8-(trifluoromethyl)quinolin-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | 29 | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50305077 (3-methyl-4-(3-(3-(methylsulfonyl)phenoxy)phenyl)-8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRbeta-LBD | Bioorg Med Chem Lett 20: 209-12 (2010) Article DOI: 10.1016/j.bmcl.2009.10.132 BindingDB Entry DOI: 10.7270/Q2KW5G4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 648 total ) | Next | Last >> |