

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50305077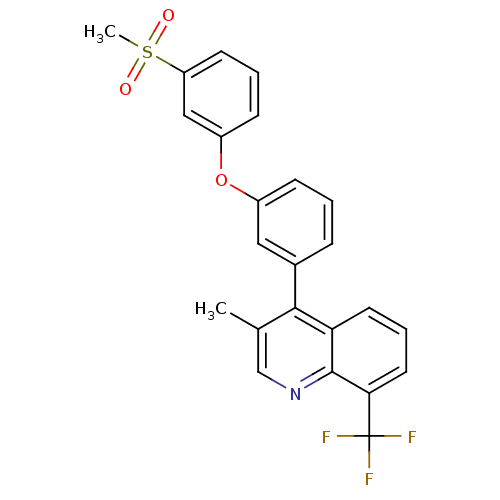 (3-methyl-4-(3-(3-(methylsulfonyl)phenoxy)phenyl)-8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]TO901317 from human recombinant LXRbeta expressed in Escherichia coli by flashplate method | Bioorg Med Chem Lett 20: 689-93 (2010) Article DOI: 10.1016/j.bmcl.2009.11.062 BindingDB Entry DOI: 10.7270/Q2W66KV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50305077 (3-methyl-4-(3-(3-(methylsulfonyl)phenoxy)phenyl)-8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRbeta-LBD | Bioorg Med Chem Lett 20: 209-12 (2010) Article DOI: 10.1016/j.bmcl.2009.10.132 BindingDB Entry DOI: 10.7270/Q2KW5G4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50305077 (3-methyl-4-(3-(3-(methylsulfonyl)phenoxy)phenyl)-8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRbeta LBD | Bioorg Med Chem Lett 20: 526-30 (2010) Article DOI: 10.1016/j.bmcl.2009.11.099 BindingDB Entry DOI: 10.7270/Q2HT2PDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50305077 (3-methyl-4-(3-(3-(methylsulfonyl)phenoxy)phenyl)-8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]T0901317 from human recombinant LXRbeta LBD | Bioorg Med Chem Lett 20: 521-5 (2010) Article DOI: 10.1016/j.bmcl.2009.11.098 BindingDB Entry DOI: 10.7270/Q27D2W3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50305077 (3-methyl-4-(3-(3-(methylsulfonyl)phenoxy)phenyl)-8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 183 | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Agonist activity at human Gal4-LBD fused LXRbeta LBD expressed in Huh7 cells by transient transactivation assay | Bioorg Med Chem Lett 20: 521-5 (2010) Article DOI: 10.1016/j.bmcl.2009.11.098 BindingDB Entry DOI: 10.7270/Q27D2W3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||