 Found 1761 hits with Last Name = 'wrobel' and Initial = 'j'
Found 1761 hits with Last Name = 'wrobel' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50243699
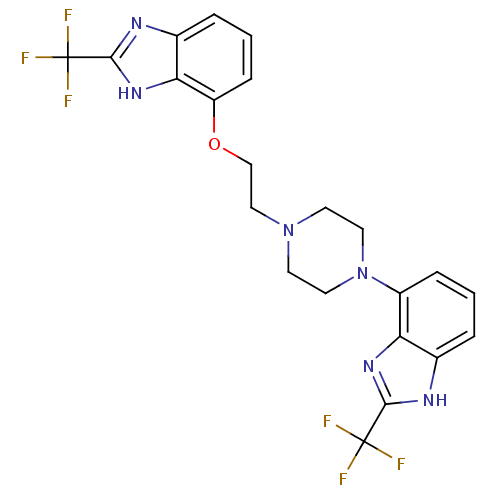
(2-(trifluoromethyl)-7-(2-(4-(2-(trifluoromethyl)-1...)Show SMILES FC(F)(F)c1nc2cccc(OCCN3CCN(CC3)c3cccc4[nH]c(nc34)C(F)(F)F)c2[nH]1 Show InChI InChI=1S/C22H20F6N6O/c23-21(24,25)19-29-13-3-1-5-15(17(13)31-19)34-9-7-33(8-10-34)11-12-35-16-6-2-4-14-18(16)32-20(30-14)22(26,27)28/h1-6H,7-12H2,(H,29,31)(H,30,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT1D receptor |
Bioorg Med Chem 16: 6617-40 (2008)
Article DOI: 10.1016/j.bmc.2008.05.024
BindingDB Entry DOI: 10.7270/Q2FB52Q7 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Binding affinity towards progesterone receptor was measured |
Bioorg Med Chem Lett 11: 2747-50 (2001)
BindingDB Entry DOI: 10.7270/Q2222V92 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50243700
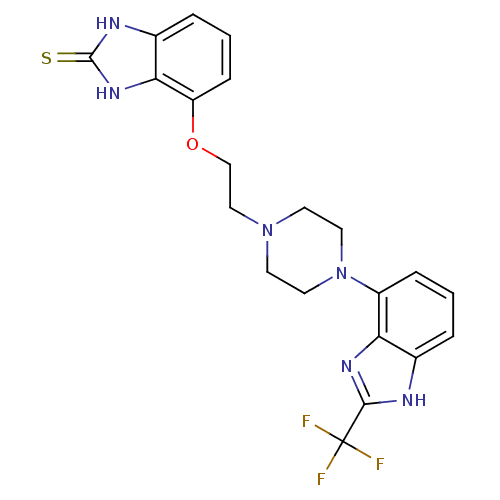
(4-(2-(4-(2-(trifluoromethyl)-1H-benzo[d]imidazol-4...)Show SMILES FC(F)(F)c1nc2c(cccc2[nH]1)N1CCN(CCOc2cccc3[nH]c(=S)[nH]c23)CC1 Show InChI InChI=1S/C21H21F3N6OS/c22-21(23,24)19-25-13-3-1-5-15(17(13)27-19)30-9-7-29(8-10-30)11-12-31-16-6-2-4-14-18(16)28-20(32)26-14/h1-6H,7-12H2,(H,25,27)(H2,26,28,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT1A receptor |
Bioorg Med Chem 16: 6617-40 (2008)
Article DOI: 10.1016/j.bmc.2008.05.024
BindingDB Entry DOI: 10.7270/Q2FB52Q7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50243699

(2-(trifluoromethyl)-7-(2-(4-(2-(trifluoromethyl)-1...)Show SMILES FC(F)(F)c1nc2cccc(OCCN3CCN(CC3)c3cccc4[nH]c(nc34)C(F)(F)F)c2[nH]1 Show InChI InChI=1S/C22H20F6N6O/c23-21(24,25)19-29-13-3-1-5-15(17(13)31-19)34-9-7-33(8-10-34)11-12-35-16-6-2-4-14-18(16)32-20(30-14)22(26,27)28/h1-6H,7-12H2,(H,29,31)(H,30,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT1A receptor |
Bioorg Med Chem 16: 6617-40 (2008)
Article DOI: 10.1016/j.bmc.2008.05.024
BindingDB Entry DOI: 10.7270/Q2FB52Q7 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50066523

(2,2,4-Trimethyl-6-(3-nitro-phenyl)-1,2-dihydro-qui...)Show SMILES CC1=CC(C)(C)Nc2ccc(cc12)-c1cccc(c1)[N+]([O-])=O |t:1| Show InChI InChI=1S/C18H18N2O2/c1-12-11-18(2,3)19-17-8-7-14(10-16(12)17)13-5-4-6-15(9-13)20(21)22/h4-11,19H,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Binding affinity towards progesterone receptor was measured |
Bioorg Med Chem Lett 11: 2747-50 (2001)
BindingDB Entry DOI: 10.7270/Q2222V92 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50105572

(1-Benzyl-6-(3-nitro-phenyl)-1,3-dihydro-benzoimida...)Show SMILES [O-][N+](=O)c1cccc(c1)-[c-]1ccc2nc(=[SH+])n(Cc3ccccc3)c2c1 Show InChI InChI=1S/C20H14N3O2S/c24-23(25)17-8-4-7-15(11-17)16-9-10-18-19(12-16)22(20(26)21-18)13-14-5-2-1-3-6-14/h1-12H,13H2/q-1/p+1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Binding affinity towards progesterone receptor was measured |
Bioorg Med Chem Lett 11: 2747-50 (2001)
BindingDB Entry DOI: 10.7270/Q2222V92 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50243699

(2-(trifluoromethyl)-7-(2-(4-(2-(trifluoromethyl)-1...)Show SMILES FC(F)(F)c1nc2cccc(OCCN3CCN(CC3)c3cccc4[nH]c(nc34)C(F)(F)F)c2[nH]1 Show InChI InChI=1S/C22H20F6N6O/c23-21(24,25)19-29-13-3-1-5-15(17(13)31-19)34-9-7-33(8-10-34)11-12-35-16-6-2-4-14-18(16)32-20(30-14)22(26,27)28/h1-6H,7-12H2,(H,29,31)(H,30,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT1B receptor |
Bioorg Med Chem 16: 6617-40 (2008)
Article DOI: 10.1016/j.bmc.2008.05.024
BindingDB Entry DOI: 10.7270/Q2FB52Q7 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50105564
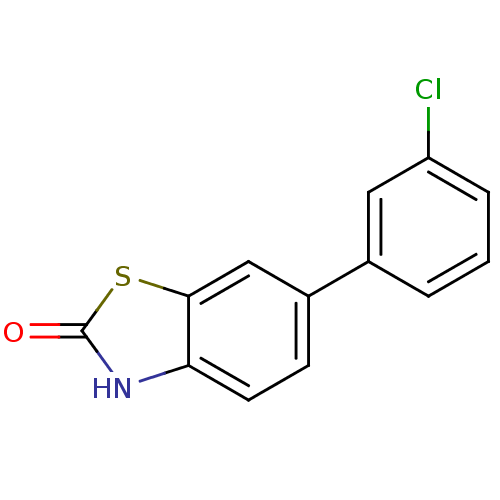
(6-(3-Chloro-phenyl)-3H-benzothiazol-2-one | CHEMBL...)Show InChI InChI=1S/C13H8ClNOS/c14-10-3-1-2-8(6-10)9-4-5-11-12(7-9)17-13(16)15-11/h1-7H,(H,15,16) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 127 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against progesterone stimulated alkaline phosphatase activity in T47D human breast carcinoma cell line |
Bioorg Med Chem Lett 11: 2747-50 (2001)
BindingDB Entry DOI: 10.7270/Q2222V92 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50105561

(1-Benzyl-6-(3-chloro-phenyl)-1,3-dihydro-benzoimid...)Show SMILES Clc1cccc(c1)-c1ccc2[nH]c(=S)n(Cc3ccccc3)c2c1 Show InChI InChI=1S/C20H15ClN2S/c21-17-8-4-7-15(11-17)16-9-10-18-19(12-16)23(20(24)22-18)13-14-5-2-1-3-6-14/h1-12H,13H2,(H,22,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Binding affinity towards progesterone receptor was measured |
Bioorg Med Chem Lett 11: 2747-50 (2001)
BindingDB Entry DOI: 10.7270/Q2222V92 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50244213
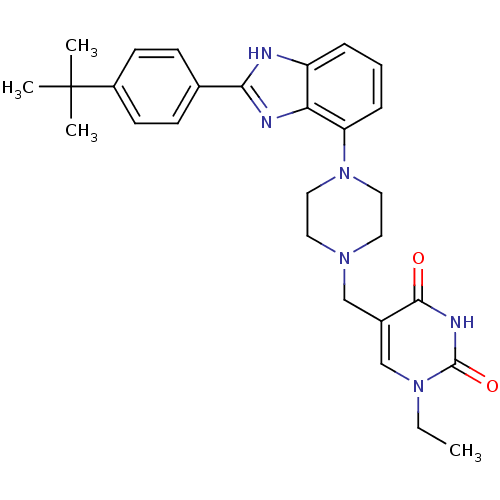
(5-((4-(2-(4-tert-butylphenyl)-1H-benzo[d]imidazol-...)Show SMILES CCn1cc(CN2CCN(CC2)c2cccc3[nH]c(nc23)-c2ccc(cc2)C(C)(C)C)c(=O)[nH]c1=O Show InChI InChI=1S/C28H34N6O2/c1-5-33-18-20(26(35)31-27(33)36)17-32-13-15-34(16-14-32)23-8-6-7-22-24(23)30-25(29-22)19-9-11-21(12-10-19)28(2,3)4/h6-12,18H,5,13-17H2,1-4H3,(H,29,30)(H,31,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT2A receptor (unknown origin) |
Bioorg Med Chem 16: 6617-40 (2008)
Article DOI: 10.1016/j.bmc.2008.05.024
BindingDB Entry DOI: 10.7270/Q2FB52Q7 |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50256882
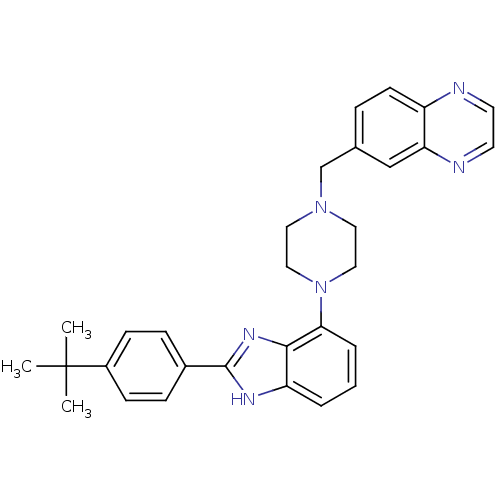
(6-((4-(2-(4-tert-butylphenyl)-1H-benzo[d]imidazol-...)Show SMILES CC(C)(C)c1ccc(cc1)-c1nc2c(cccc2[nH]1)N1CCN(Cc2ccc3nccnc3c2)CC1 Show InChI InChI=1S/C30H32N6/c1-30(2,3)23-10-8-22(9-11-23)29-33-25-5-4-6-27(28(25)34-29)36-17-15-35(16-18-36)20-21-7-12-24-26(19-21)32-14-13-31-24/h4-14,19H,15-18,20H2,1-3H3,(H,33,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human neurokinin NK2 receptor |
J Med Chem 52: 2148-52 (2009)
Article DOI: 10.1021/jm801572m
BindingDB Entry DOI: 10.7270/Q2ZC82RT |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50105566

(6-(3-Nitro-phenyl)-3H-benzothiazol-2-one | CHEMBL3...)Show SMILES [O-][N+](=O)c1cccc(c1)-c1ccc2[n-]c(=[OH+])sc2c1 Show InChI InChI=1S/C13H8N2O3S/c16-13-14-11-5-4-9(7-12(11)19-13)8-2-1-3-10(6-8)15(17)18/h1-7H,(H,14,16) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 332 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Binding affinity towards progesterone receptor was measured |
Bioorg Med Chem Lett 11: 2747-50 (2001)
BindingDB Entry DOI: 10.7270/Q2222V92 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50105563

(1-Benzyl-6-(3-chloro-phenyl)-1,3-dihydro-benzoimid...)Show SMILES Clc1cccc(c1)-c1ccc2[nH]c(=O)n(Cc3ccccc3)c2c1 Show InChI InChI=1S/C20H15ClN2O/c21-17-8-4-7-15(11-17)16-9-10-18-19(12-16)23(20(24)22-18)13-14-5-2-1-3-6-14/h1-12H,13H2,(H,22,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 521 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Binding affinity towards progesterone receptor was measured |
Bioorg Med Chem Lett 11: 2747-50 (2001)
BindingDB Entry DOI: 10.7270/Q2222V92 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50105560

(1-Isopropyl-6-(3-nitro-phenyl)-1,3-dihydro-benzoim...)Show SMILES CC(C)n1c2c[c-](ccc2nc1=[OH+])-c1cccc(c1)[N+]([O-])=O Show InChI InChI=1S/C16H14N3O3/c1-10(2)18-15-9-12(6-7-14(15)17-16(18)20)11-4-3-5-13(8-11)19(21)22/h3-10H,1-2H3/q-1/p+1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 525 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Binding affinity towards progesterone receptor was measured |
Bioorg Med Chem Lett 11: 2747-50 (2001)
BindingDB Entry DOI: 10.7270/Q2222V92 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50105565

(6-(3-Chloro-phenyl)-1-isopropyl-1,3-dihydro-benzoi...)Show InChI InChI=1S/C16H15ClN2O/c1-10(2)19-15-9-12(6-7-14(15)18-16(19)20)11-4-3-5-13(17)8-11/h3-10H,1-2H3,(H,18,20) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 525 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Binding affinity towards progesterone receptor was measured |
Bioorg Med Chem Lett 11: 2747-50 (2001)
BindingDB Entry DOI: 10.7270/Q2222V92 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50105562
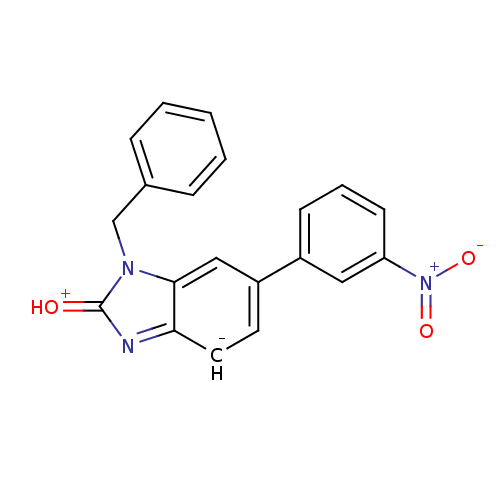
(1-Benzyl-6-(3-nitro-phenyl)-1,3-dihydro-benzoimida...)Show SMILES [O-][N+](=O)c1cccc(c1)-[c-]1ccc2nc(=[OH+])n(Cc3ccccc3)c2c1 Show InChI InChI=1S/C20H14N3O3/c24-20-21-18-10-9-16(15-7-4-8-17(11-15)23(25)26)12-19(18)22(20)13-14-5-2-1-3-6-14/h1-12H,13H2/q-1/p+1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 714 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Binding affinity towards progesterone receptor was measured |
Bioorg Med Chem Lett 11: 2747-50 (2001)
BindingDB Entry DOI: 10.7270/Q2222V92 |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50256882

(6-((4-(2-(4-tert-butylphenyl)-1H-benzo[d]imidazol-...)Show SMILES CC(C)(C)c1ccc(cc1)-c1nc2c(cccc2[nH]1)N1CCN(Cc2ccc3nccnc3c2)CC1 Show InChI InChI=1S/C30H32N6/c1-30(2,3)23-10-8-22(9-11-23)29-33-25-5-4-6-27(28(25)34-29)36-17-15-35(16-18-36)20-21-7-12-24-26(19-21)32-14-13-31-24/h4-14,19H,15-18,20H2,1-3H3,(H,33,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H2 receptor |
J Med Chem 52: 2148-52 (2009)
Article DOI: 10.1021/jm801572m
BindingDB Entry DOI: 10.7270/Q2ZC82RT |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50105559

(1-Benzyl-5-(3-chloro-phenyl)-1,3-dihydro-benzoimid...)Show SMILES Clc1cccc(c1)-c1ccc2n(Cc3ccccc3)c(=O)[nH]c2c1 Show InChI InChI=1S/C20H15ClN2O/c21-17-8-4-7-15(11-17)16-9-10-19-18(12-16)22-20(24)23(19)13-14-5-2-1-3-6-14/h1-12H,13H2,(H,22,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Binding affinity towards progesterone receptor was measured |
Bioorg Med Chem Lett 11: 2747-50 (2001)
BindingDB Entry DOI: 10.7270/Q2222V92 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50105567

(1-Benzyl-5-(3-nitro-phenyl)-1,3-dihydro-benzoimida...)Show SMILES [O-][N+](=O)c1cccc(c1)-c1cc[c-]2n(Cc3ccccc3)c(=[OH+])nc2c1 Show InChI InChI=1S/C20H14N3O3/c24-20-21-18-12-16(15-7-4-8-17(11-15)23(25)26)9-10-19(18)22(20)13-14-5-2-1-3-6-14/h1-12H,13H2/q-1/p+1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against progesterone stimulated alkaline phosphatase activity in T47D human breast carcinoma cell line |
Bioorg Med Chem Lett 11: 2747-50 (2001)
BindingDB Entry DOI: 10.7270/Q2222V92 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50105568
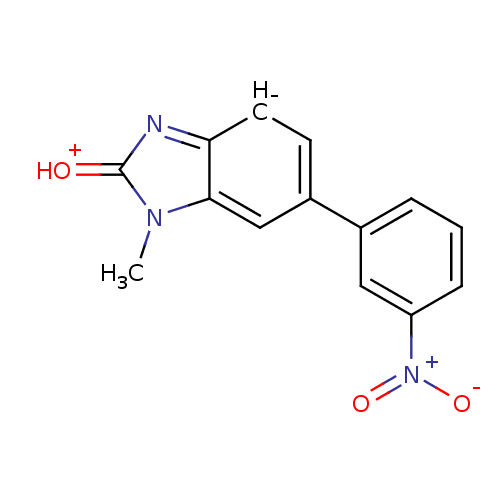
(1-Methyl-6-(3-nitro-phenyl)-1,3-dihydro-benzoimida...)Show SMILES Cn1c2c[c-](ccc2nc1=[OH+])-c1cccc(c1)[N+]([O-])=O Show InChI InChI=1S/C14H10N3O3/c1-16-13-8-10(5-6-12(13)15-14(16)18)9-3-2-4-11(7-9)17(19)20/h2-8H,1H3/q-1/p+1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Binding affinity towards progesterone receptor was measured |
Bioorg Med Chem Lett 11: 2747-50 (2001)
BindingDB Entry DOI: 10.7270/Q2222V92 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Progesterone receptor antagonist activity based on its ability to block progesterone induced alkaline phosphatase in the human breast cancer cell lin... |
Bioorg Med Chem Lett 12: 3487-90 (2002)
BindingDB Entry DOI: 10.7270/Q2R210RC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Progesterone receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Women's Health Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity against progesterone receptor (PR) in an alkaline phosphatase assay in the T47D human breast carcinoma cell line |
J Med Chem 45: 4379-82 (2002)
BindingDB Entry DOI: 10.7270/Q22F7P5W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Progesterone receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Antagonist activity at progesterone receptor assessed as progesterone-induced alkaline phosphatase activity in human T47D cells |
Bioorg Med Chem Lett 18: 5015-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.015
BindingDB Entry DOI: 10.7270/Q25B03D7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Progesterone receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against progesterone stimulated alkaline phosphatase activity in T47D human breast carcinoma cell line |
Bioorg Med Chem Lett 11: 2747-50 (2001)
BindingDB Entry DOI: 10.7270/Q2222V92 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Progesterone receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Antagonist activity at human PR expressed in human T47D cells assessed as inhibition of progesterone induced alkaline phosphatase |
J Med Chem 51: 1861-73 (2008)
Article DOI: 10.1021/jm701080t
BindingDB Entry DOI: 10.7270/Q2G44R56 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Progesterone receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of progesterone receptor mediated progesterone-induced alkaline phosphatase activity in human T47D cells |
Bioorg Med Chem 16: 6589-600 (2008)
Article DOI: 10.1016/j.bmc.2008.05.018
BindingDB Entry DOI: 10.7270/Q2V125QZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Progesterone receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Antagonistic activity against Progesterone receptor (PR) in transcriptional activation assay in human T47D breast carcinoma cell line |
Bioorg Med Chem Lett 12: 787-90 (2002)
BindingDB Entry DOI: 10.7270/Q2WS8TSW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50375423
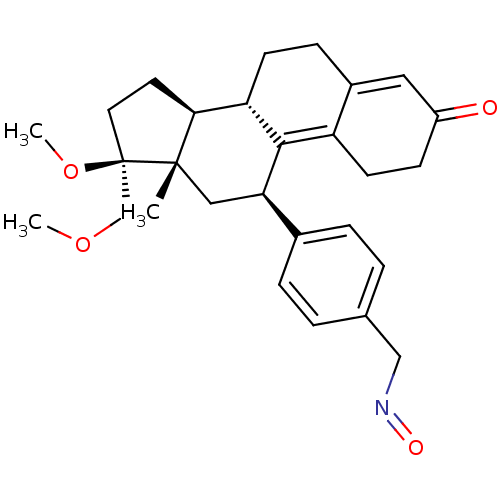
(ASOPRISNIL)Show SMILES COC[C@@]1(CC[C@H]2[C@@H]3CCC4=CC(=O)CCC4=C3[C@H](C[C@]12C)c1ccc(CN=O)cc1)OC |r,c:17,t:10| Show InChI InChI=1S/C28H35NO4/c1-27-15-24(19-6-4-18(5-7-19)16-29-31)26-22-11-9-21(30)14-20(22)8-10-23(26)25(27)12-13-28(27,33-3)17-32-2/h4-7,14,23-25H,8-13,15-17H2,1-3H3/t23-,24+,25-,27-,28+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity against progesterone receptor in human T47D cells by alkaline phosphatase assay |
Bioorg Med Chem Lett 22: 7119-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.077
BindingDB Entry DOI: 10.7270/Q2S183NW |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against progesterone induced PRE-luciferase activity in CV-cells |
Bioorg Med Chem Lett 11: 2747-50 (2001)
BindingDB Entry DOI: 10.7270/Q2222V92 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50375423

(ASOPRISNIL)Show SMILES COC[C@@]1(CC[C@H]2[C@@H]3CCC4=CC(=O)CCC4=C3[C@H](C[C@]12C)c1ccc(CN=O)cc1)OC |r,c:17,t:10| Show InChI InChI=1S/C28H35NO4/c1-27-15-24(19-6-4-18(5-7-19)16-29-31)26-22-11-9-21(30)14-20(22)8-10-23(26)25(27)12-13-28(27,33-3)17-32-2/h4-7,14,23-25H,8-13,15-17H2,1-3H3/t23-,24+,25-,27-,28+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at progesterone receptor by cellular reporter gene assay |
Bioorg Med Chem Lett 22: 7119-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.09.077
BindingDB Entry DOI: 10.7270/Q2S183NW |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Women's Health Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity against the Progesterone Receptor (PR) |
J Med Chem 45: 4379-82 (2002)
BindingDB Entry DOI: 10.7270/Q22F7P5W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Progesterone receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Women's Health Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity against progesterone receptor (PR) using PRE-luciferase plasmid co-transfected CV-1 cells |
J Med Chem 45: 4379-82 (2002)
BindingDB Entry DOI: 10.7270/Q22F7P5W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50219260
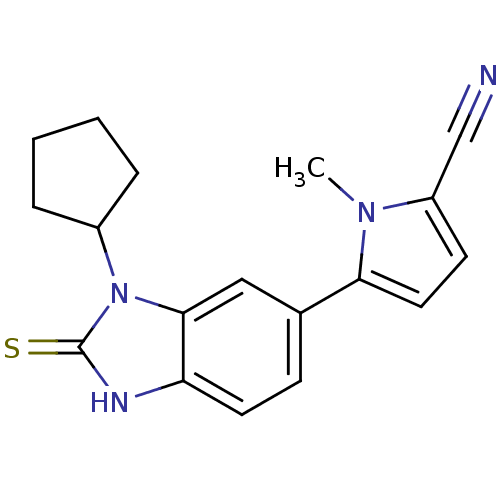
(5-(3-cyclopentyl-2-thioxo-2,3-dihydro-1H-benzimida...)Show SMILES Cn1c(ccc1-c1ccc2[nH]c(=S)n(C3CCCC3)c2c1)C#N Show InChI InChI=1S/C18H18N4S/c1-21-14(11-19)7-9-16(21)12-6-8-15-17(10-12)22(18(23)20-15)13-4-2-3-5-13/h6-10,13H,2-5H2,1H3,(H,20,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity to cytosolic PR in T47D cells by competition binding assay |
Bioorg Med Chem 15: 6556-64 (2007)
Article DOI: 10.1016/j.bmc.2007.07.011
BindingDB Entry DOI: 10.7270/Q2CV4HGN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50335231
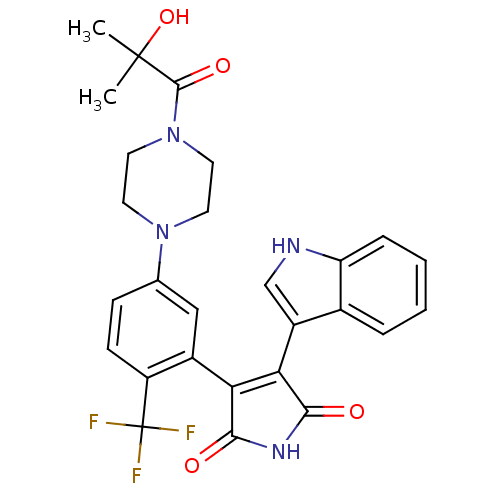
(3-{5-[4-(2-Hydroxy-2-methyl-propionyl)-piperazin-1...)Show SMILES CC(C)(O)C(=O)N1CCN(CC1)c1ccc(c(c1)C1=C(C(=O)NC1=O)c1c[nH]c2ccccc12)C(F)(F)F |t:20| Show InChI InChI=1S/C27H25F3N4O4/c1-26(2,38)25(37)34-11-9-33(10-12-34)15-7-8-19(27(28,29)30)17(13-15)21-22(24(36)32-23(21)35)18-14-31-20-6-4-3-5-16(18)20/h3-8,13-14,31,38H,9-12H2,1-2H3,(H,32,35,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Fox Chase Chemical Diversity Center, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK3 (unknown origin) |
Bioorg Med Chem Lett 24: 1116-21 (2014)
Article DOI: 10.1016/j.bmcl.2014.01.001
BindingDB Entry DOI: 10.7270/Q2PZ5B9J |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50375821
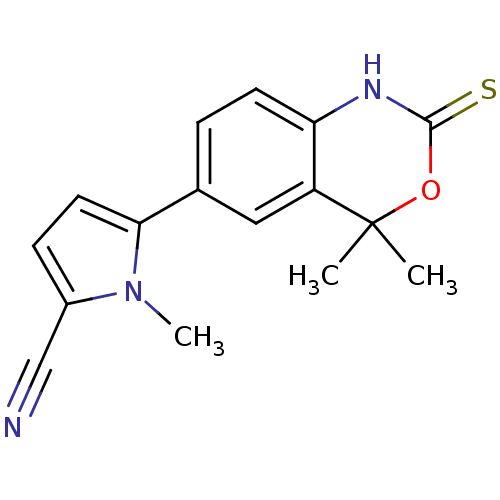
(TANAPROGET)Show InChI InChI=1S/C16H15N3OS/c1-16(2)12-8-10(4-6-13(12)18-15(21)20-16)14-7-5-11(9-17)19(14)3/h4-8H,1-3H3,(H,18,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]R5020 from human PR in human T47D cells by whole cell assay |
J Med Chem 51: 1861-73 (2008)
Article DOI: 10.1021/jm701080t
BindingDB Entry DOI: 10.7270/Q2G44R56 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Antagonist activity at glucocorticoid receptor by Gal4-DNA binding domain-hormone receptor LBD one-hybrid assay |
Bioorg Med Chem Lett 18: 5015-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.015
BindingDB Entry DOI: 10.7270/Q25B03D7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Progesterone receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]R5020 from human PR in human T47D cells by whole cell assay |
J Med Chem 51: 1861-73 (2008)
Article DOI: 10.1021/jm701080t
BindingDB Entry DOI: 10.7270/Q2G44R56 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Antagonist activity at cloned glucocorticoid receptor-ligand binding domain expressed in african green monkey COS7 cells by GAL4 luciferase reporter ... |
Bioorg Med Chem 16: 6589-600 (2008)
Article DOI: 10.1016/j.bmc.2008.05.018
BindingDB Entry DOI: 10.7270/Q2V125QZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM50355501

(INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...)Show SMILES N#CC[C@H](C1CCCC1)n1cc(cn1)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C17H18N6/c18-7-5-15(12-3-1-2-4-12)23-10-13(9-22-23)16-14-6-8-19-17(14)21-11-20-16/h6,8-12,15H,1-5H2,(H,19,20,21)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Fox Chase Chemical Diversity Center, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK1 (unknown origin) |
Bioorg Med Chem Lett 24: 1116-21 (2014)
Article DOI: 10.1016/j.bmcl.2014.01.001
BindingDB Entry DOI: 10.7270/Q2PZ5B9J |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Antagonist activity at human GR ligand binding domain expressed in african green monkey COS7 cells in presence of Dexamethasone by Gal4 hybrid assay |
J Med Chem 51: 1861-73 (2008)
Article DOI: 10.1021/jm701080t
BindingDB Entry DOI: 10.7270/Q2G44R56 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50355501

(INCB-018424 | RUXOLITINIB | RUXOLITINIB PHOSPHATE ...)Show SMILES N#CC[C@H](C1CCCC1)n1cc(cn1)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C17H18N6/c18-7-5-15(12-3-1-2-4-12)23-10-13(9-22-23)16-14-6-8-19-17(14)21-11-20-16/h6,8-12,15H,1-5H2,(H,19,20,21)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Fox Chase Chemical Diversity Center, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 (unknown origin) |
Bioorg Med Chem Lett 24: 1116-21 (2014)
Article DOI: 10.1016/j.bmcl.2014.01.001
BindingDB Entry DOI: 10.7270/Q2PZ5B9J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50306072

(2-isobutyl-1-(3-(3-(methylsulfonyl)phenoxy)phenyl)...)Show SMILES CC(C)Cc1nc2c(cccc2n1-c1cccc(Oc2cccc(c2)S(C)(=O)=O)c1)C(F)(F)F Show InChI InChI=1S/C25H23F3N2O3S/c1-16(2)13-23-29-24-21(25(26,27)28)11-6-12-22(24)30(23)17-7-4-8-18(14-17)33-19-9-5-10-20(15-19)34(3,31)32/h4-12,14-16H,13H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRalpha LBD |
Bioorg Med Chem Lett 20: 526-30 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.099
BindingDB Entry DOI: 10.7270/Q2HT2PDZ |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Women's Health Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity against the Glucocorticoid Receptor (GR) |
J Med Chem 45: 4379-82 (2002)
BindingDB Entry DOI: 10.7270/Q22F7P5W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50378590
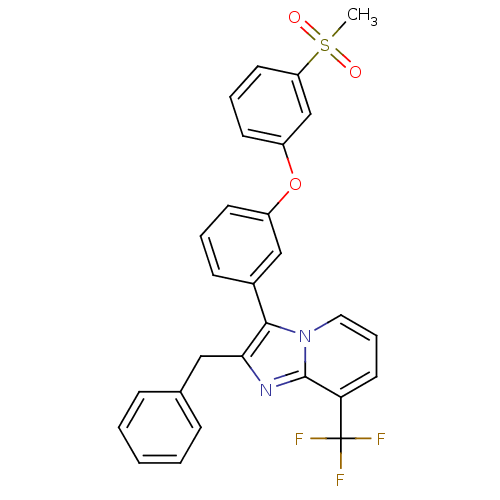
(CHEMBL611735)Show SMILES CS(=O)(=O)c1cccc(Oc2cccc(c2)-c2c(Cc3ccccc3)nc3c(cccn23)C(F)(F)F)c1 Show InChI InChI=1S/C28H21F3N2O3S/c1-37(34,35)23-13-6-12-22(18-23)36-21-11-5-10-20(17-21)26-25(16-19-8-3-2-4-9-19)32-27-24(28(29,30)31)14-7-15-33(26)27/h2-15,17-18H,16H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRalpha LBD |
Bioorg Med Chem Lett 20: 521-5 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.098
BindingDB Entry DOI: 10.7270/Q27D2W3S |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50305075
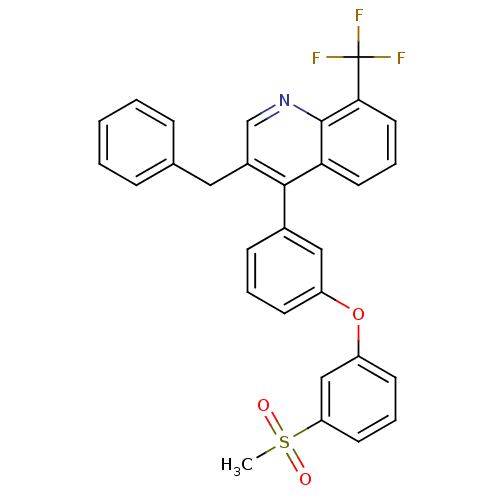
(3-benzyl-4-(3-(3-(methylsulfonyl)phenoxy)phenyl)-8...)Show SMILES CS(=O)(=O)c1cccc(Oc2cccc(c2)-c2c(Cc3ccccc3)cnc3c(cccc23)C(F)(F)F)c1 Show InChI InChI=1S/C30H22F3NO3S/c1-38(35,36)25-13-6-12-24(18-25)37-23-11-5-10-21(17-23)28-22(16-20-8-3-2-4-9-20)19-34-29-26(28)14-7-15-27(29)30(31,32)33/h2-15,17-19H,16H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRalpha-LBD |
Bioorg Med Chem Lett 20: 209-12 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.132
BindingDB Entry DOI: 10.7270/Q2KW5G4B |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50305077

(3-methyl-4-(3-(3-(methylsulfonyl)phenoxy)phenyl)-8...)Show SMILES Cc1cnc2c(cccc2c1-c1cccc(Oc2cccc(c2)S(C)(=O)=O)c1)C(F)(F)F Show InChI InChI=1S/C24H18F3NO3S/c1-15-14-28-23-20(10-5-11-21(23)24(25,26)27)22(15)16-6-3-7-17(12-16)31-18-8-4-9-19(13-18)32(2,29)30/h3-14H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRalpha LBD |
Bioorg Med Chem Lett 20: 526-30 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.099
BindingDB Entry DOI: 10.7270/Q2HT2PDZ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50306070
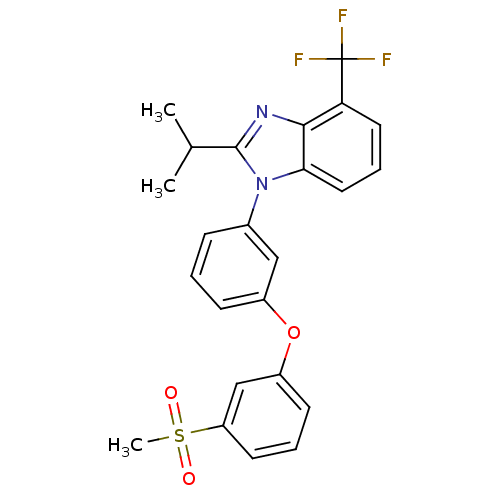
(2-isopropyl-1-(3-(3-(methylsulfonyl)phenoxy)phenyl...)Show SMILES CC(C)c1nc2c(cccc2n1-c1cccc(Oc2cccc(c2)S(C)(=O)=O)c1)C(F)(F)F Show InChI InChI=1S/C24H21F3N2O3S/c1-15(2)23-28-22-20(24(25,26)27)11-6-12-21(22)29(23)16-7-4-8-17(13-16)32-18-9-5-10-19(14-18)33(3,30)31/h4-15H,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRalpha LBD |
Bioorg Med Chem Lett 20: 526-30 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.099
BindingDB Entry DOI: 10.7270/Q2HT2PDZ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50305077

(3-methyl-4-(3-(3-(methylsulfonyl)phenoxy)phenyl)-8...)Show SMILES Cc1cnc2c(cccc2c1-c1cccc(Oc2cccc(c2)S(C)(=O)=O)c1)C(F)(F)F Show InChI InChI=1S/C24H18F3NO3S/c1-15-14-28-23-20(10-5-11-21(23)24(25,26)27)22(15)16-6-3-7-17(12-16)31-18-8-4-9-19(13-18)32(2,29)30/h3-14H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRalpha-LBD |
Bioorg Med Chem Lett 20: 209-12 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.132
BindingDB Entry DOI: 10.7270/Q2KW5G4B |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50305077

(3-methyl-4-(3-(3-(methylsulfonyl)phenoxy)phenyl)-8...)Show SMILES Cc1cnc2c(cccc2c1-c1cccc(Oc2cccc(c2)S(C)(=O)=O)c1)C(F)(F)F Show InChI InChI=1S/C24H18F3NO3S/c1-15-14-28-23-20(10-5-11-21(23)24(25,26)27)22(15)16-6-3-7-17(12-16)31-18-8-4-9-19(13-18)32(2,29)30/h3-14H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]TO901317 from human recombinant LXRbeta expressed in Escherichia coli by flashplate method |
Bioorg Med Chem Lett 20: 689-93 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.062
BindingDB Entry DOI: 10.7270/Q2W66KV8 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50305077

(3-methyl-4-(3-(3-(methylsulfonyl)phenoxy)phenyl)-8...)Show SMILES Cc1cnc2c(cccc2c1-c1cccc(Oc2cccc(c2)S(C)(=O)=O)c1)C(F)(F)F Show InChI InChI=1S/C24H18F3NO3S/c1-15-14-28-23-20(10-5-11-21(23)24(25,26)27)22(15)16-6-3-7-17(12-16)31-18-8-4-9-19(13-18)32(2,29)30/h3-14H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRalpha LBD |
Bioorg Med Chem Lett 20: 521-5 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.098
BindingDB Entry DOI: 10.7270/Q27D2W3S |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data