 Found 782 hits with Last Name = 'feingold' and Initial = 'i'
Found 782 hits with Last Name = 'feingold' and Initial = 'i' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50243699
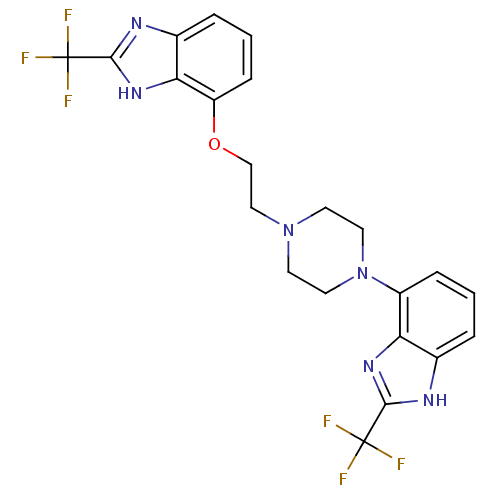
(2-(trifluoromethyl)-7-(2-(4-(2-(trifluoromethyl)-1...)Show SMILES FC(F)(F)c1nc2cccc(OCCN3CCN(CC3)c3cccc4[nH]c(nc34)C(F)(F)F)c2[nH]1 Show InChI InChI=1S/C22H20F6N6O/c23-21(24,25)19-29-13-3-1-5-15(17(13)31-19)34-9-7-33(8-10-34)11-12-35-16-6-2-4-14-18(16)32-20(30-14)22(26,27)28/h1-6H,7-12H2,(H,29,31)(H,30,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT1D receptor |
Bioorg Med Chem 16: 6617-40 (2008)
Article DOI: 10.1016/j.bmc.2008.05.024
BindingDB Entry DOI: 10.7270/Q2FB52Q7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50243700
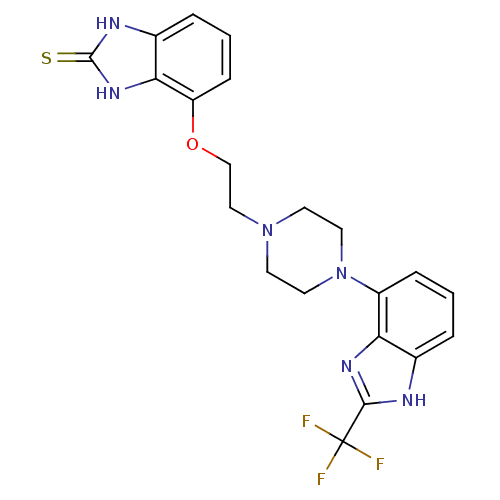
(4-(2-(4-(2-(trifluoromethyl)-1H-benzo[d]imidazol-4...)Show SMILES FC(F)(F)c1nc2c(cccc2[nH]1)N1CCN(CCOc2cccc3[nH]c(=S)[nH]c23)CC1 Show InChI InChI=1S/C21H21F3N6OS/c22-21(23,24)19-25-13-3-1-5-15(17(13)27-19)30-9-7-29(8-10-30)11-12-31-16-6-2-4-14-18(16)28-20(32)26-14/h1-6H,7-12H2,(H,25,27)(H2,26,28,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT1A receptor |
Bioorg Med Chem 16: 6617-40 (2008)
Article DOI: 10.1016/j.bmc.2008.05.024
BindingDB Entry DOI: 10.7270/Q2FB52Q7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50243699

(2-(trifluoromethyl)-7-(2-(4-(2-(trifluoromethyl)-1...)Show SMILES FC(F)(F)c1nc2cccc(OCCN3CCN(CC3)c3cccc4[nH]c(nc34)C(F)(F)F)c2[nH]1 Show InChI InChI=1S/C22H20F6N6O/c23-21(24,25)19-29-13-3-1-5-15(17(13)31-19)34-9-7-33(8-10-34)11-12-35-16-6-2-4-14-18(16)32-20(30-14)22(26,27)28/h1-6H,7-12H2,(H,29,31)(H,30,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT1A receptor |
Bioorg Med Chem 16: 6617-40 (2008)
Article DOI: 10.1016/j.bmc.2008.05.024
BindingDB Entry DOI: 10.7270/Q2FB52Q7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50243699

(2-(trifluoromethyl)-7-(2-(4-(2-(trifluoromethyl)-1...)Show SMILES FC(F)(F)c1nc2cccc(OCCN3CCN(CC3)c3cccc4[nH]c(nc34)C(F)(F)F)c2[nH]1 Show InChI InChI=1S/C22H20F6N6O/c23-21(24,25)19-29-13-3-1-5-15(17(13)31-19)34-9-7-33(8-10-34)11-12-35-16-6-2-4-14-18(16)32-20(30-14)22(26,27)28/h1-6H,7-12H2,(H,29,31)(H,30,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT1B receptor |
Bioorg Med Chem 16: 6617-40 (2008)
Article DOI: 10.1016/j.bmc.2008.05.024
BindingDB Entry DOI: 10.7270/Q2FB52Q7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50244213
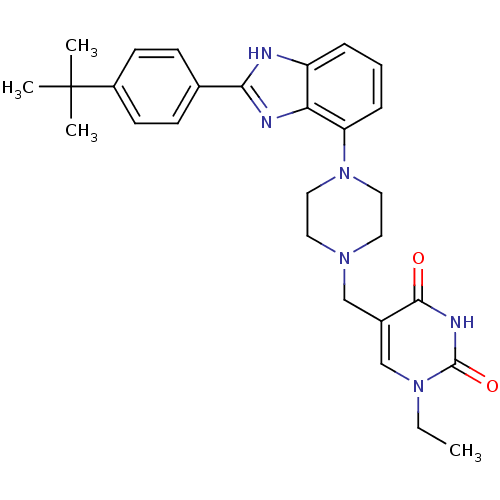
(5-((4-(2-(4-tert-butylphenyl)-1H-benzo[d]imidazol-...)Show SMILES CCn1cc(CN2CCN(CC2)c2cccc3[nH]c(nc23)-c2ccc(cc2)C(C)(C)C)c(=O)[nH]c1=O Show InChI InChI=1S/C28H34N6O2/c1-5-33-18-20(26(35)31-27(33)36)17-32-13-15-34(16-14-32)23-8-6-7-22-24(23)30-25(29-22)19-9-11-21(12-10-19)28(2,3)4/h6-12,18H,5,13-17H2,1-4H3,(H,29,30)(H,31,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity to 5HT2A receptor (unknown origin) |
Bioorg Med Chem 16: 6617-40 (2008)
Article DOI: 10.1016/j.bmc.2008.05.024
BindingDB Entry DOI: 10.7270/Q2FB52Q7 |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50256882
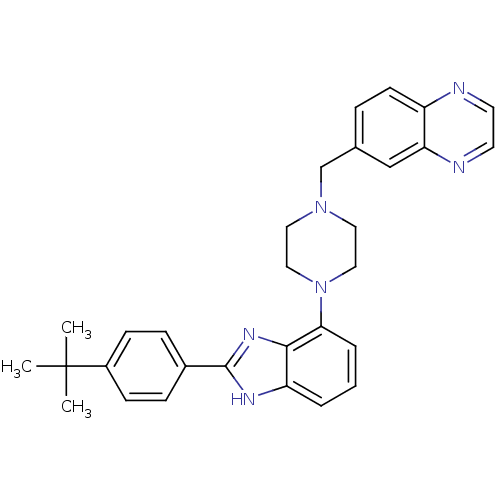
(6-((4-(2-(4-tert-butylphenyl)-1H-benzo[d]imidazol-...)Show SMILES CC(C)(C)c1ccc(cc1)-c1nc2c(cccc2[nH]1)N1CCN(Cc2ccc3nccnc3c2)CC1 Show InChI InChI=1S/C30H32N6/c1-30(2,3)23-10-8-22(9-11-23)29-33-25-5-4-6-27(28(25)34-29)36-17-15-35(16-18-36)20-21-7-12-24-26(19-21)32-14-13-31-24/h4-14,19H,15-18,20H2,1-3H3,(H,33,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human neurokinin NK2 receptor |
J Med Chem 52: 2148-52 (2009)
Article DOI: 10.1021/jm801572m
BindingDB Entry DOI: 10.7270/Q2ZC82RT |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50256882

(6-((4-(2-(4-tert-butylphenyl)-1H-benzo[d]imidazol-...)Show SMILES CC(C)(C)c1ccc(cc1)-c1nc2c(cccc2[nH]1)N1CCN(Cc2ccc3nccnc3c2)CC1 Show InChI InChI=1S/C30H32N6/c1-30(2,3)23-10-8-22(9-11-23)29-33-25-5-4-6-27(28(25)34-29)36-17-15-35(16-18-36)20-21-7-12-24-26(19-21)32-14-13-31-24/h4-14,19H,15-18,20H2,1-3H3,(H,33,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human histamine H2 receptor |
J Med Chem 52: 2148-52 (2009)
Article DOI: 10.1021/jm801572m
BindingDB Entry DOI: 10.7270/Q2ZC82RT |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50306072

(2-isobutyl-1-(3-(3-(methylsulfonyl)phenoxy)phenyl)...)Show SMILES CC(C)Cc1nc2c(cccc2n1-c1cccc(Oc2cccc(c2)S(C)(=O)=O)c1)C(F)(F)F Show InChI InChI=1S/C25H23F3N2O3S/c1-16(2)13-23-29-24-21(25(26,27)28)11-6-12-22(24)30(23)17-7-4-8-18(14-17)33-19-9-5-10-20(15-19)34(3,31)32/h4-12,14-16H,13H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRalpha LBD |
Bioorg Med Chem Lett 20: 526-30 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.099
BindingDB Entry DOI: 10.7270/Q2HT2PDZ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50378590
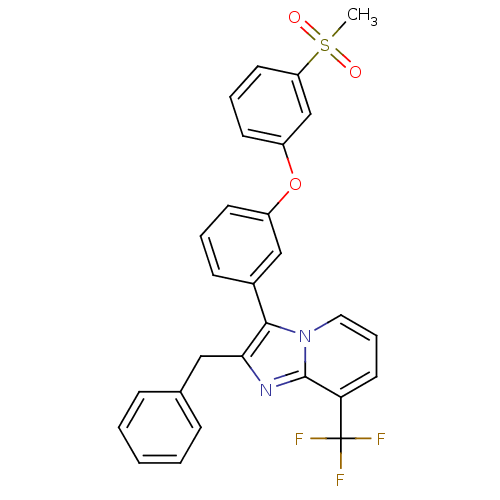
(CHEMBL611735)Show SMILES CS(=O)(=O)c1cccc(Oc2cccc(c2)-c2c(Cc3ccccc3)nc3c(cccn23)C(F)(F)F)c1 Show InChI InChI=1S/C28H21F3N2O3S/c1-37(34,35)23-13-6-12-22(18-23)36-21-11-5-10-20(17-21)26-25(16-19-8-3-2-4-9-19)32-27-24(28(29,30)31)14-7-15-33(26)27/h2-15,17-18H,16H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRalpha LBD |
Bioorg Med Chem Lett 20: 521-5 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.098
BindingDB Entry DOI: 10.7270/Q27D2W3S |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50305077

(3-methyl-4-(3-(3-(methylsulfonyl)phenoxy)phenyl)-8...)Show SMILES Cc1cnc2c(cccc2c1-c1cccc(Oc2cccc(c2)S(C)(=O)=O)c1)C(F)(F)F Show InChI InChI=1S/C24H18F3NO3S/c1-15-14-28-23-20(10-5-11-21(23)24(25,26)27)22(15)16-6-3-7-17(12-16)31-18-8-4-9-19(13-18)32(2,29)30/h3-14H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRalpha LBD |
Bioorg Med Chem Lett 20: 521-5 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.098
BindingDB Entry DOI: 10.7270/Q27D2W3S |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50306070
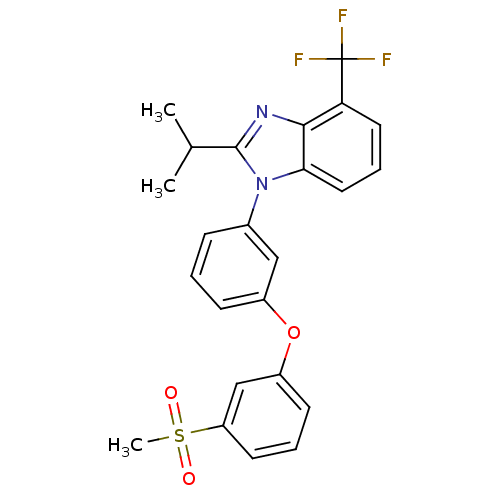
(2-isopropyl-1-(3-(3-(methylsulfonyl)phenoxy)phenyl...)Show SMILES CC(C)c1nc2c(cccc2n1-c1cccc(Oc2cccc(c2)S(C)(=O)=O)c1)C(F)(F)F Show InChI InChI=1S/C24H21F3N2O3S/c1-15(2)23-28-22-20(24(25,26)27)11-6-12-21(22)29(23)16-7-4-8-17(13-16)32-18-9-5-10-19(14-18)33(3,30)31/h4-15H,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRalpha LBD |
Bioorg Med Chem Lett 20: 526-30 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.099
BindingDB Entry DOI: 10.7270/Q2HT2PDZ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50305077

(3-methyl-4-(3-(3-(methylsulfonyl)phenoxy)phenyl)-8...)Show SMILES Cc1cnc2c(cccc2c1-c1cccc(Oc2cccc(c2)S(C)(=O)=O)c1)C(F)(F)F Show InChI InChI=1S/C24H18F3NO3S/c1-15-14-28-23-20(10-5-11-21(23)24(25,26)27)22(15)16-6-3-7-17(12-16)31-18-8-4-9-19(13-18)32(2,29)30/h3-14H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRalpha LBD |
Bioorg Med Chem Lett 20: 526-30 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.099
BindingDB Entry DOI: 10.7270/Q2HT2PDZ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50305077

(3-methyl-4-(3-(3-(methylsulfonyl)phenoxy)phenyl)-8...)Show SMILES Cc1cnc2c(cccc2c1-c1cccc(Oc2cccc(c2)S(C)(=O)=O)c1)C(F)(F)F Show InChI InChI=1S/C24H18F3NO3S/c1-15-14-28-23-20(10-5-11-21(23)24(25,26)27)22(15)16-6-3-7-17(12-16)31-18-8-4-9-19(13-18)32(2,29)30/h3-14H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]TO901317 from human recombinant LXRbeta expressed in Escherichia coli by flashplate method |
Bioorg Med Chem Lett 20: 689-93 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.062
BindingDB Entry DOI: 10.7270/Q2W66KV8 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50306073
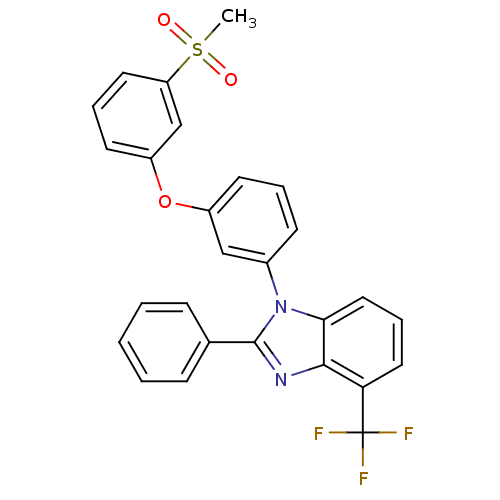
(1-(3-(3-(methylsulfonyl)phenoxy)phenyl)-2-phenyl-4...)Show SMILES CS(=O)(=O)c1cccc(Oc2cccc(c2)-n2c(nc3c(cccc23)C(F)(F)F)-c2ccccc2)c1 Show InChI InChI=1S/C27H19F3N2O3S/c1-36(33,34)22-13-6-12-21(17-22)35-20-11-5-10-19(16-20)32-24-15-7-14-23(27(28,29)30)25(24)31-26(32)18-8-3-2-4-9-18/h2-17H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRalpha LBD |
Bioorg Med Chem Lett 20: 526-30 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.099
BindingDB Entry DOI: 10.7270/Q2HT2PDZ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50306074

(2-(4-fluorobenzyl)-1-(3-(3-(methylsulfonyl)phenoxy...)Show SMILES CS(=O)(=O)c1cccc(Oc2cccc(c2)-n2c(Cc3ccc(F)cc3)nc3c(cccc23)C(F)(F)F)c1 Show InChI InChI=1S/C28H20F4N2O3S/c1-38(35,36)23-8-3-7-22(17-23)37-21-6-2-5-20(16-21)34-25-10-4-9-24(28(30,31)32)27(25)33-26(34)15-18-11-13-19(29)14-12-18/h2-14,16-17H,15H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRalpha LBD |
Bioorg Med Chem Lett 20: 526-30 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.099
BindingDB Entry DOI: 10.7270/Q2HT2PDZ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50306068
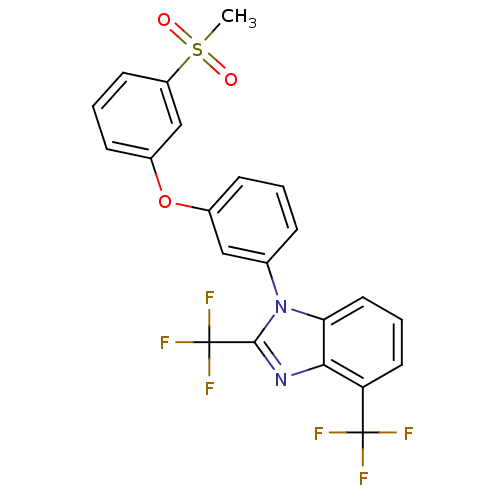
(1-(3-(3-(methylsulfonyl)phenoxy)phenyl)-2,4-bis(tr...)Show SMILES CS(=O)(=O)c1cccc(Oc2cccc(c2)-n2c(nc3c(cccc23)C(F)(F)F)C(F)(F)F)c1 Show InChI InChI=1S/C22H14F6N2O3S/c1-34(31,32)16-8-3-7-15(12-16)33-14-6-2-5-13(11-14)30-18-10-4-9-17(21(23,24)25)19(18)29-20(30)22(26,27)28/h2-12H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRalpha LBD |
Bioorg Med Chem Lett 20: 526-30 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.099
BindingDB Entry DOI: 10.7270/Q2HT2PDZ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta [154-461]
(Homo sapiens (Human)) | BDBM20015

(2-[4-({[3-(3-benzyl-8-chloroquinolin-4-yl)phenyl]a...)Show SMILES OC(=O)Cc1ccc(CNc2cccc(c2)-c2c(Cc3ccccc3)cnc3c(Cl)cccc23)cc1 Show InChI InChI=1S/C31H25ClN2O2/c32-28-11-5-10-27-30(25(20-34-31(27)28)16-21-6-2-1-3-7-21)24-8-4-9-26(18-24)33-19-23-14-12-22(13-15-23)17-29(35)36/h1-15,18,20,33H,16-17,19H2,(H,35,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | 23 | n/a | n/a | n/a | n/a |
Wyeth Research
| Assay Description
Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... |
Bioorg Med Chem 15: 3321-33 (2007)
Article DOI: 10.1016/j.bmc.2007.03.013
BindingDB Entry DOI: 10.7270/Q2VH5M37 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta [154-461]
(Homo sapiens (Human)) | BDBM20024
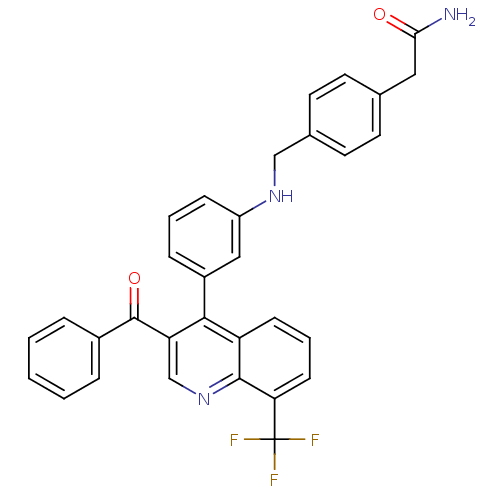
(2-{4-[({3-[3-benzoyl-8-(trifluoromethyl)quinolin-4...)Show SMILES NC(=O)Cc1ccc(CNc2cccc(c2)-c2c(cnc3c(cccc23)C(F)(F)F)C(=O)c2ccccc2)cc1 Show InChI InChI=1S/C32H24F3N3O2/c33-32(34,35)27-11-5-10-25-29(26(19-38-30(25)27)31(40)22-6-2-1-3-7-22)23-8-4-9-24(17-23)37-18-21-14-12-20(13-15-21)16-28(36)39/h1-15,17,19,37H,16,18H2,(H2,36,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | 316 | n/a | n/a | n/a | n/a |
Wyeth Research
| Assay Description
Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... |
Bioorg Med Chem 15: 3321-33 (2007)
Article DOI: 10.1016/j.bmc.2007.03.013
BindingDB Entry DOI: 10.7270/Q2VH5M37 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50306075
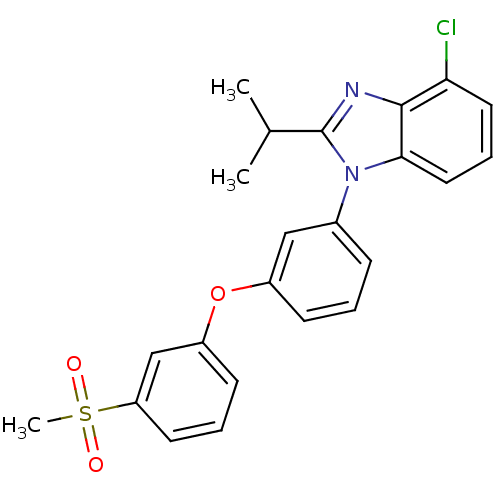
(4-chloro-2-isopropyl-1-(3-(3-(methylsulfonyl)pheno...)Show SMILES CC(C)c1nc2c(Cl)cccc2n1-c1cccc(Oc2cccc(c2)S(C)(=O)=O)c1 Show InChI InChI=1S/C23H21ClN2O3S/c1-15(2)23-25-22-20(24)11-6-12-21(22)26(23)16-7-4-8-17(13-16)29-18-9-5-10-19(14-18)30(3,27)28/h4-15H,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRalpha LBD |
Bioorg Med Chem Lett 20: 526-30 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.099
BindingDB Entry DOI: 10.7270/Q2HT2PDZ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50317735
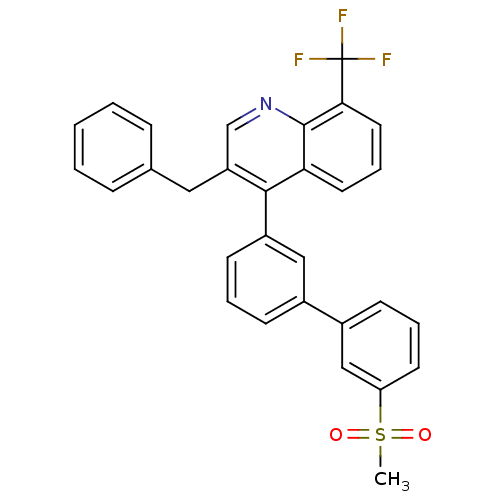
(3-benzyl-4-(3'-(methylsulfonyl)biphenyl-3-yl)-8-(t...)Show SMILES CS(=O)(=O)c1cccc(c1)-c1cccc(c1)-c1c(Cc2ccccc2)cnc2c(cccc12)C(F)(F)F Show InChI InChI=1S/C30H22F3NO2S/c1-37(35,36)25-13-6-11-22(18-25)21-10-5-12-23(17-21)28-24(16-20-8-3-2-4-9-20)19-34-29-26(28)14-7-15-27(29)30(31,32)33/h2-15,17-19H,16H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from LXRbeta ligand binding domain |
Bioorg Med Chem Lett 20: 2903-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.031
BindingDB Entry DOI: 10.7270/Q2TD9XJZ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50306069

(2-ethyl-1-(3-(3-(methylsulfonyl)phenoxy)phenyl)-4-...)Show SMILES CCc1nc2c(cccc2n1-c1cccc(Oc2cccc(c2)S(C)(=O)=O)c1)C(F)(F)F Show InChI InChI=1S/C23H19F3N2O3S/c1-3-21-27-22-19(23(24,25)26)11-6-12-20(22)28(21)15-7-4-8-16(13-15)31-17-9-5-10-18(14-17)32(2,29)30/h4-14H,3H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRalpha LBD |
Bioorg Med Chem Lett 20: 526-30 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.099
BindingDB Entry DOI: 10.7270/Q2HT2PDZ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50317744
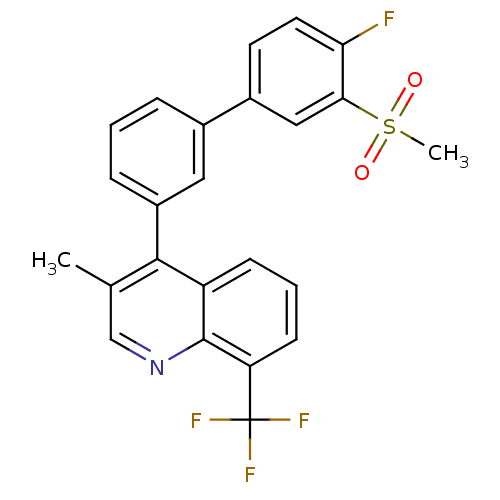
(4-(4'-fluoro-3'-(methylsulfonyl)biphenyl-3-yl)-3-m...)Show SMILES Cc1cnc2c(cccc2c1-c1cccc(c1)-c1ccc(F)c(c1)S(C)(=O)=O)C(F)(F)F Show InChI InChI=1S/C24H17F4NO2S/c1-14-13-29-23-18(7-4-8-19(23)24(26,27)28)22(14)17-6-3-5-15(11-17)16-9-10-20(25)21(12-16)32(2,30)31/h3-13H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from LXRbeta ligand binding domain |
Bioorg Med Chem Lett 20: 2903-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.031
BindingDB Entry DOI: 10.7270/Q2TD9XJZ |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50244213

(5-((4-(2-(4-tert-butylphenyl)-1H-benzo[d]imidazol-...)Show SMILES CCn1cc(CN2CCN(CC2)c2cccc3[nH]c(nc23)-c2ccc(cc2)C(C)(C)C)c(=O)[nH]c1=O Show InChI InChI=1S/C28H34N6O2/c1-5-33-18-20(26(35)31-27(33)36)17-32-13-15-34(16-14-32)23-8-6-7-22-24(23)30-25(29-22)19-9-11-21(12-10-19)28(2,3)4/h6-12,18H,5,13-17H2,1-4H3,(H,29,30)(H,31,35,36) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [125I]-(D-Trp6)LHRH from human recombinant GnRH receptor |
Bioorg Med Chem 16: 6617-40 (2008)
Article DOI: 10.1016/j.bmc.2008.05.024
BindingDB Entry DOI: 10.7270/Q2FB52Q7 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50244213

(5-((4-(2-(4-tert-butylphenyl)-1H-benzo[d]imidazol-...)Show SMILES CCn1cc(CN2CCN(CC2)c2cccc3[nH]c(nc23)-c2ccc(cc2)C(C)(C)C)c(=O)[nH]c1=O Show InChI InChI=1S/C28H34N6O2/c1-5-33-18-20(26(35)31-27(33)36)17-32-13-15-34(16-14-32)23-8-6-7-22-24(23)30-25(29-22)19-9-11-21(12-10-19)28(2,3)4/h6-12,18H,5,13-17H2,1-4H3,(H,29,30)(H,31,35,36) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [125I]-(D-Trp6)-GnRH from human GnRH receptor |
Bioorg Med Chem Lett 19: 1986-90 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.043
BindingDB Entry DOI: 10.7270/Q28S4PS4 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50256836
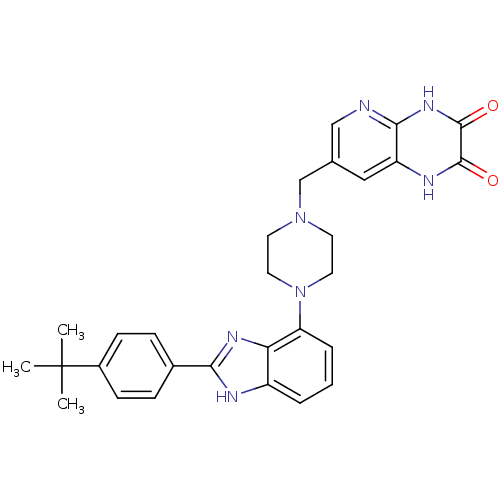
(7-((4-(2-(4-tert-butylphenyl)-1H-benzo[d]imidazol-...)Show SMILES CC(C)(C)c1ccc(cc1)-c1nc2c(cccc2[nH]1)N1CCN(Cc2cnc3[nH]c(=O)c(=O)[nH]c3c2)CC1 Show InChI InChI=1S/C29H31N7O2/c1-29(2,3)20-9-7-19(8-10-20)25-31-21-5-4-6-23(24(21)33-25)36-13-11-35(12-14-36)17-18-15-22-26(30-16-18)34-28(38)27(37)32-22/h4-10,15-16H,11-14,17H2,1-3H3,(H,31,33)(H,32,37)(H,30,34,38) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [D-Trp6]-GnRH from human recombinant GnRH receptor |
J Med Chem 52: 2148-52 (2009)
Article DOI: 10.1021/jm801572m
BindingDB Entry DOI: 10.7270/Q2ZC82RT |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50317733
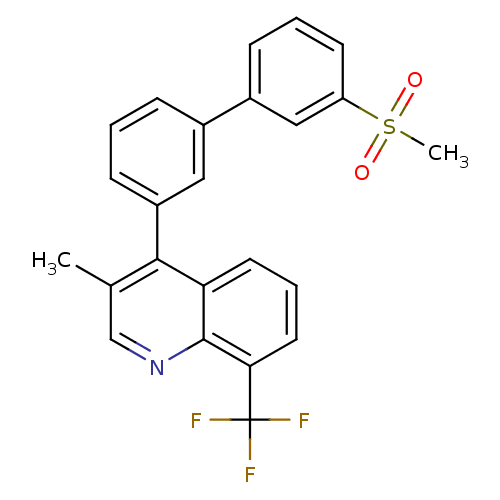
(3-methyl-4-(3'-(methylsulfonyl)biphenyl-3-yl)-8-(t...)Show SMILES Cc1cnc2c(cccc2c1-c1cccc(c1)-c1cccc(c1)S(C)(=O)=O)C(F)(F)F Show InChI InChI=1S/C24H18F3NO2S/c1-15-14-28-23-20(10-5-11-21(23)24(25,26)27)22(15)18-8-3-6-16(12-18)17-7-4-9-19(13-17)31(2,29)30/h3-14H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from LXRbeta ligand binding domain |
Bioorg Med Chem Lett 20: 2903-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.031
BindingDB Entry DOI: 10.7270/Q2TD9XJZ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta [154-461]
(Homo sapiens (Human)) | BDBM20001
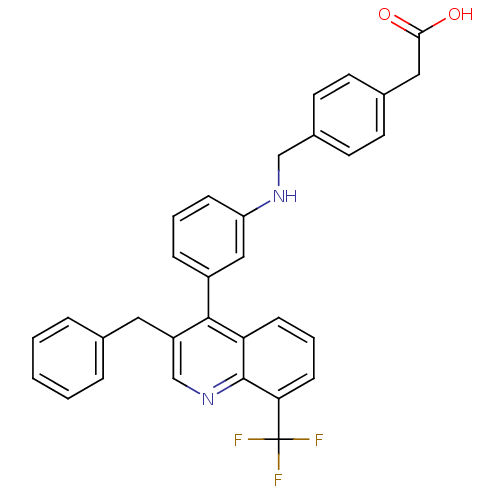
(2-{4-[({3-[3-benzyl-8-(trifluoromethyl)quinolin-4-...)Show SMILES OC(=O)Cc1ccc(CNc2cccc(c2)-c2c(Cc3ccccc3)cnc3c(cccc23)C(F)(F)F)cc1 Show InChI InChI=1S/C32H25F3N2O2/c33-32(34,35)28-11-5-10-27-30(25(20-37-31(27)28)16-21-6-2-1-3-7-21)24-8-4-9-26(18-24)36-19-23-14-12-22(13-15-23)17-29(38)39/h1-15,18,20,36H,16-17,19H2,(H,38,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | 33 | n/a | n/a | n/a | n/a |
Wyeth Research
| Assay Description
Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... |
Bioorg Med Chem 15: 3321-33 (2007)
Article DOI: 10.1016/j.bmc.2007.03.013
BindingDB Entry DOI: 10.7270/Q2VH5M37 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta [154-461]
(Homo sapiens (Human)) | BDBM20001

(2-{4-[({3-[3-benzyl-8-(trifluoromethyl)quinolin-4-...)Show SMILES OC(=O)Cc1ccc(CNc2cccc(c2)-c2c(Cc3ccccc3)cnc3c(cccc23)C(F)(F)F)cc1 Show InChI InChI=1S/C32H25F3N2O2/c33-32(34,35)28-11-5-10-27-30(25(20-37-31(27)28)16-21-6-2-1-3-7-21)24-8-4-9-26(18-24)36-19-23-14-12-22(13-15-23)17-29(38)39/h1-15,18,20,36H,16-17,19H2,(H,38,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | 33 | n/a | n/a | 7.4 | 4 |
Wyeth Research
| Assay Description
Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... |
J Med Chem 49: 6151-4 (2006)
Article DOI: 10.1021/jm0609566
BindingDB Entry DOI: 10.7270/Q20863KH |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50378593
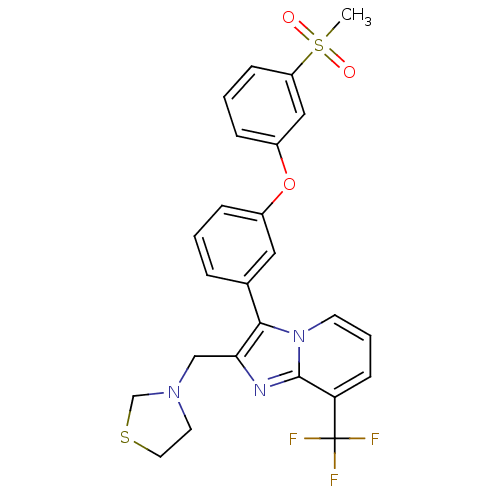
(CHEMBL612007)Show SMILES CS(=O)(=O)c1cccc(Oc2cccc(c2)-c2c(CN3CCSC3)nc3c(cccn23)C(F)(F)F)c1 Show InChI InChI=1S/C25H22F3N3O3S2/c1-36(32,33)20-8-3-7-19(14-20)34-18-6-2-5-17(13-18)23-22(15-30-11-12-35-16-30)29-24-21(25(26,27)28)9-4-10-31(23)24/h2-10,13-14H,11-12,15-16H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRalpha LBD |
Bioorg Med Chem Lett 20: 521-5 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.098
BindingDB Entry DOI: 10.7270/Q27D2W3S |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50305077

(3-methyl-4-(3-(3-(methylsulfonyl)phenoxy)phenyl)-8...)Show SMILES Cc1cnc2c(cccc2c1-c1cccc(Oc2cccc(c2)S(C)(=O)=O)c1)C(F)(F)F Show InChI InChI=1S/C24H18F3NO3S/c1-15-14-28-23-20(10-5-11-21(23)24(25,26)27)22(15)16-6-3-7-17(12-16)31-18-8-4-9-19(13-18)32(2,29)30/h3-14H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]TO901317 from human recombinant LXRalpha expressed in Escherichia coli by flashplate method |
Bioorg Med Chem Lett 20: 689-93 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.062
BindingDB Entry DOI: 10.7270/Q2W66KV8 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta [154-461]
(Homo sapiens (Human)) | BDBM20000
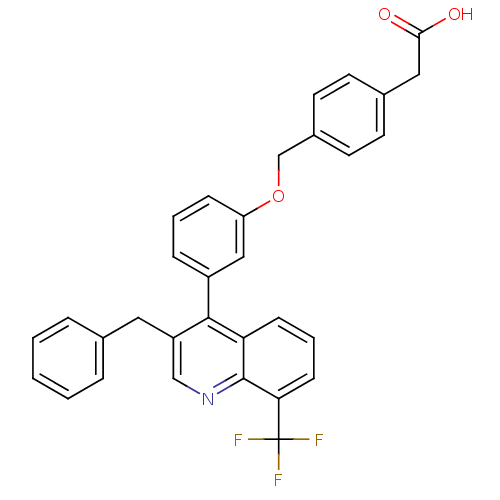
(2-(4-{3-[3-benzyl-8-(trifluoromethyl)quinolin-4-yl...)Show SMILES OC(=O)Cc1ccc(COc2cccc(c2)-c2c(Cc3ccccc3)cnc3c(cccc23)C(F)(F)F)cc1 Show InChI InChI=1S/C32H24F3NO3/c33-32(34,35)28-11-5-10-27-30(25(19-36-31(27)28)16-21-6-2-1-3-7-21)24-8-4-9-26(18-24)39-20-23-14-12-22(13-15-23)17-29(37)38/h1-15,18-19H,16-17,20H2,(H,37,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | 90 | n/a | n/a | 7.4 | 4 |
Wyeth Research
| Assay Description
Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... |
J Med Chem 51: 7161-8 (2008)
Article DOI: 10.1021/jm800799q
BindingDB Entry DOI: 10.7270/Q2XW4H4G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Oxysterols receptor LXR-beta [154-461]
(Homo sapiens (Human)) | BDBM20001

(2-{4-[({3-[3-benzyl-8-(trifluoromethyl)quinolin-4-...)Show SMILES OC(=O)Cc1ccc(CNc2cccc(c2)-c2c(Cc3ccccc3)cnc3c(cccc23)C(F)(F)F)cc1 Show InChI InChI=1S/C32H25F3N2O2/c33-32(34,35)28-11-5-10-27-30(25(20-37-31(27)28)16-21-6-2-1-3-7-21)24-8-4-9-26(18-24)36-19-23-14-12-22(13-15-23)17-29(38)39/h1-15,18,20,36H,16-17,19H2,(H,38,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | 90 | n/a | n/a | 7.4 | 4 |
Wyeth Research
| Assay Description
Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... |
J Med Chem 51: 7161-8 (2008)
Article DOI: 10.1021/jm800799q
BindingDB Entry DOI: 10.7270/Q2XW4H4G |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50305501
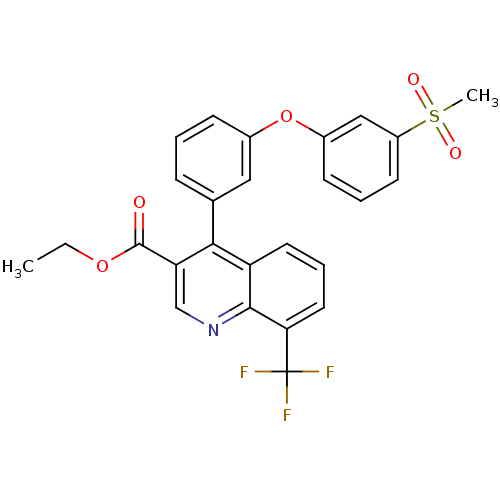
(CHEMBL590098 | ethyl 4-(3-(3-(methylsulfonyl)pheno...)Show SMILES CCOC(=O)c1cnc2c(cccc2c1-c1cccc(Oc2cccc(c2)S(C)(=O)=O)c1)C(F)(F)F Show InChI InChI=1S/C26H20F3NO5S/c1-3-34-25(31)21-15-30-24-20(11-6-12-22(24)26(27,28)29)23(21)16-7-4-8-17(13-16)35-18-9-5-10-19(14-18)36(2,32)33/h4-15H,3H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]TO901317 from human recombinant LXRbeta expressed in Escherichia coli by flashplate method |
Bioorg Med Chem Lett 20: 689-93 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.062
BindingDB Entry DOI: 10.7270/Q2W66KV8 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta [154-461]
(Homo sapiens (Human)) | BDBM20000

(2-(4-{3-[3-benzyl-8-(trifluoromethyl)quinolin-4-yl...)Show SMILES OC(=O)Cc1ccc(COc2cccc(c2)-c2c(Cc3ccccc3)cnc3c(cccc23)C(F)(F)F)cc1 Show InChI InChI=1S/C32H24F3NO3/c33-32(34,35)28-11-5-10-27-30(25(19-36-31(27)28)16-21-6-2-1-3-7-21)24-8-4-9-26(18-24)39-20-23-14-12-22(13-15-23)17-29(37)38/h1-15,18-19H,16-17,20H2,(H,37,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.10 | n/a | 71 | n/a | n/a | 7.4 | 4 |
Wyeth Research
| Assay Description
Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... |
J Med Chem 49: 6151-4 (2006)
Article DOI: 10.1021/jm0609566
BindingDB Entry DOI: 10.7270/Q20863KH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50305499
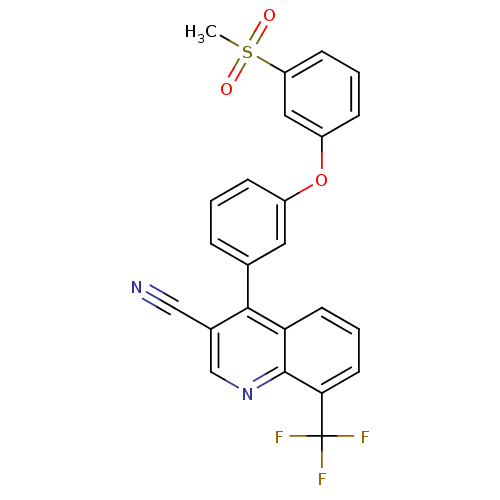
(4-(3-(3-(methylsulfonyl)phenoxy)phenyl)-8-(trifluo...)Show SMILES CS(=O)(=O)c1cccc(Oc2cccc(c2)-c2c(cnc3c(cccc23)C(F)(F)F)C#N)c1 Show InChI InChI=1S/C24H15F3N2O3S/c1-33(30,31)19-8-3-7-18(12-19)32-17-6-2-5-15(11-17)22-16(13-28)14-29-23-20(22)9-4-10-21(23)24(25,26)27/h2-12,14H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]TO901317 from human recombinant LXRbeta expressed in Escherichia coli by flashplate method |
Bioorg Med Chem Lett 20: 689-93 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.062
BindingDB Entry DOI: 10.7270/Q2W66KV8 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50315277

(5-((4-(2-(4-tert-butylphenyl)imidazo[1,2-a]pyridin...)Show SMILES CCn1cc(CN2CCN(CC2)c2cccn3cc(nc23)-c2ccc(cc2)C(C)(C)C)c(=O)[nH]c1=O Show InChI InChI=1S/C28H34N6O2/c1-5-32-18-21(26(35)30-27(32)36)17-31-13-15-33(16-14-31)24-7-6-12-34-19-23(29-25(24)34)20-8-10-22(11-9-20)28(2,3)4/h6-12,18-19H,5,13-17H2,1-4H3,(H,30,35,36) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [125I]D-Trp6-GnRH from human recombinant GNRH receptor by scintillation counting |
Bioorg Med Chem Lett 20: 2512-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.099
BindingDB Entry DOI: 10.7270/Q2W0962G |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50243853
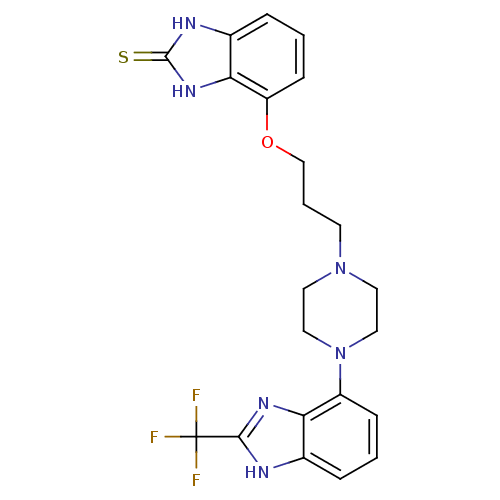
(4-(3-(4-(2-(Trifluoromethyl)-1H-benzo[d]imidazol-4...)Show SMILES FC(F)(F)c1nc2c(cccc2[nH]1)N1CCN(CCCOc2cccc3[nH]c(=S)[nH]c23)CC1 Show InChI InChI=1S/C22H23F3N6OS/c23-22(24,25)20-26-14-4-1-6-16(18(14)28-20)31-11-9-30(10-12-31)8-3-13-32-17-7-2-5-15-19(17)29-21(33)27-15/h1-2,4-7H,3,8-13H2,(H,26,28)(H2,27,29,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT1A receptor |
Bioorg Med Chem 16: 6617-40 (2008)
Article DOI: 10.1016/j.bmc.2008.05.024
BindingDB Entry DOI: 10.7270/Q2FB52Q7 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50305077

(3-methyl-4-(3-(3-(methylsulfonyl)phenoxy)phenyl)-8...)Show SMILES Cc1cnc2c(cccc2c1-c1cccc(Oc2cccc(c2)S(C)(=O)=O)c1)C(F)(F)F Show InChI InChI=1S/C24H18F3NO3S/c1-15-14-28-23-20(10-5-11-21(23)24(25,26)27)22(15)16-6-3-7-17(12-16)31-18-8-4-9-19(13-18)32(2,29)30/h3-14H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRbeta LBD |
Bioorg Med Chem Lett 20: 521-5 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.098
BindingDB Entry DOI: 10.7270/Q27D2W3S |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50306077
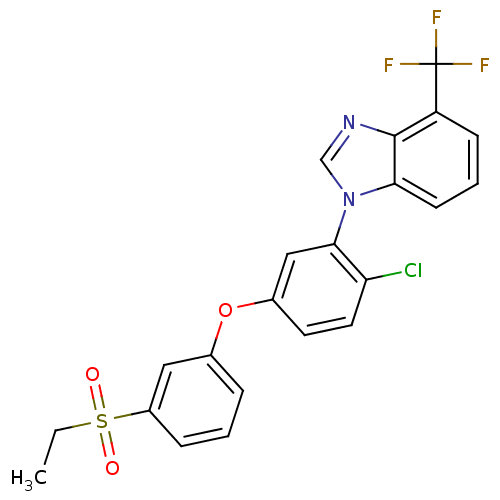
(1-(2-chloro-5-(3-(ethylsulfonyl)phenoxy)phenyl)-4-...)Show SMILES CCS(=O)(=O)c1cccc(Oc2ccc(Cl)c(c2)-n2cnc3c(cccc23)C(F)(F)F)c1 Show InChI InChI=1S/C22H16ClF3N2O3S/c1-2-32(29,30)16-6-3-5-14(11-16)31-15-9-10-18(23)20(12-15)28-13-27-21-17(22(24,25)26)7-4-8-19(21)28/h3-13H,2H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRalpha LBD |
Bioorg Med Chem Lett 20: 526-30 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.099
BindingDB Entry DOI: 10.7270/Q2HT2PDZ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta [154-461]
(Homo sapiens (Human)) | BDBM20004
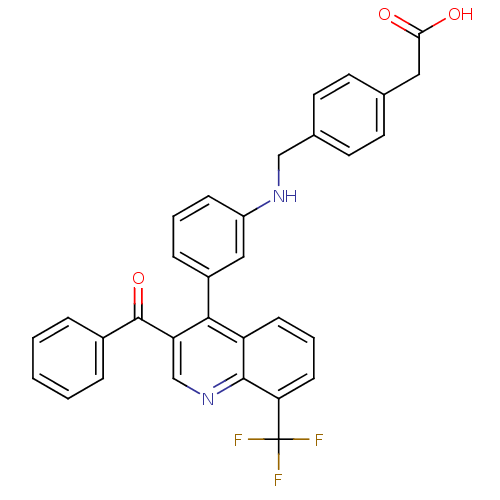
(2-{4-[({3-[3-benzoyl-8-(trifluoromethyl)quinolin-4...)Show SMILES OC(=O)Cc1ccc(CNc2cccc(c2)-c2c(cnc3c(cccc23)C(F)(F)F)C(=O)c2ccccc2)cc1 Show InChI InChI=1S/C32H23F3N2O3/c33-32(34,35)27-11-5-10-25-29(26(19-37-30(25)27)31(40)22-6-2-1-3-7-22)23-8-4-9-24(17-23)36-18-21-14-12-20(13-15-21)16-28(38)39/h1-15,17,19,36H,16,18H2,(H,38,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | 29 | n/a | n/a | n/a | n/a |
Wyeth Research
| Assay Description
Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... |
Bioorg Med Chem 15: 3321-33 (2007)
Article DOI: 10.1016/j.bmc.2007.03.013
BindingDB Entry DOI: 10.7270/Q2VH5M37 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50317737
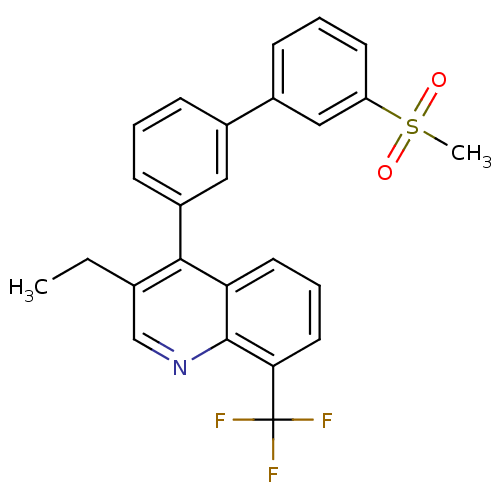
(3-ethyl-4-(3'-(methylsulfonyl)biphenyl-3-yl)-8-(tr...)Show SMILES CCc1cnc2c(cccc2c1-c1cccc(c1)-c1cccc(c1)S(C)(=O)=O)C(F)(F)F Show InChI InChI=1S/C25H20F3NO2S/c1-3-16-15-29-24-21(11-6-12-22(24)25(26,27)28)23(16)19-9-4-7-17(13-19)18-8-5-10-20(14-18)32(2,30)31/h4-15H,3H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from LXRbeta ligand binding domain |
Bioorg Med Chem Lett 20: 2903-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.031
BindingDB Entry DOI: 10.7270/Q2TD9XJZ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50305077

(3-methyl-4-(3-(3-(methylsulfonyl)phenoxy)phenyl)-8...)Show SMILES Cc1cnc2c(cccc2c1-c1cccc(Oc2cccc(c2)S(C)(=O)=O)c1)C(F)(F)F Show InChI InChI=1S/C24H18F3NO3S/c1-15-14-28-23-20(10-5-11-21(23)24(25,26)27)22(15)16-6-3-7-17(12-16)31-18-8-4-9-19(13-18)32(2,29)30/h3-14H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRbeta LBD |
Bioorg Med Chem Lett 20: 526-30 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.099
BindingDB Entry DOI: 10.7270/Q2HT2PDZ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50317746
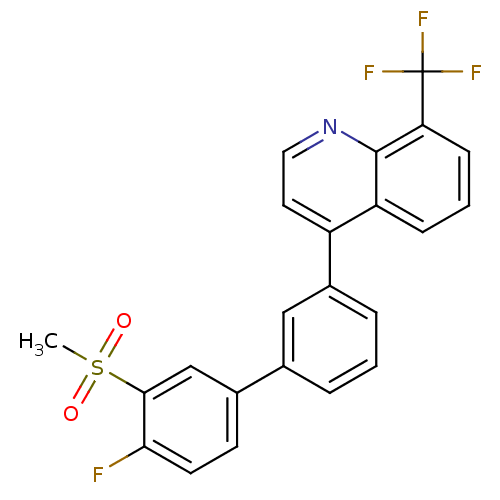
(4-(4'-fluoro-3'-(methylsulfonyl)biphenyl-3-yl)-8-(...)Show SMILES CS(=O)(=O)c1cc(ccc1F)-c1cccc(c1)-c1ccnc2c(cccc12)C(F)(F)F Show InChI InChI=1S/C23H15F4NO2S/c1-31(29,30)21-13-15(8-9-20(21)24)14-4-2-5-16(12-14)17-10-11-28-22-18(17)6-3-7-19(22)23(25,26)27/h2-13H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from LXRbeta ligand binding domain |
Bioorg Med Chem Lett 20: 2903-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.031
BindingDB Entry DOI: 10.7270/Q2TD9XJZ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50306074

(2-(4-fluorobenzyl)-1-(3-(3-(methylsulfonyl)phenoxy...)Show SMILES CS(=O)(=O)c1cccc(Oc2cccc(c2)-n2c(Cc3ccc(F)cc3)nc3c(cccc23)C(F)(F)F)c1 Show InChI InChI=1S/C28H20F4N2O3S/c1-38(35,36)23-8-3-7-22(17-23)37-21-6-2-5-20(16-21)34-25-10-4-9-24(28(30,31)32)27(25)33-26(34)15-18-11-13-19(29)14-12-18/h2-14,16-17H,15H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRbeta LBD |
Bioorg Med Chem Lett 20: 526-30 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.099
BindingDB Entry DOI: 10.7270/Q2HT2PDZ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta [154-461]
(Homo sapiens (Human)) | BDBM20020
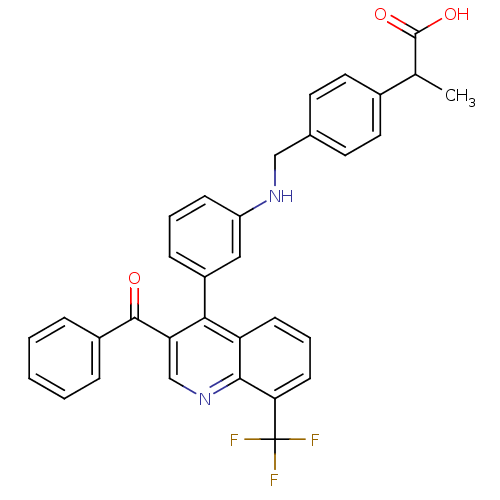
(2-{4-[({3-[3-benzoyl-8-(trifluoromethyl)quinolin-4...)Show SMILES CC(C(O)=O)c1ccc(CNc2cccc(c2)-c2c(cnc3c(cccc23)C(F)(F)F)C(=O)c2ccccc2)cc1 Show InChI InChI=1S/C33H25F3N2O3/c1-20(32(40)41)22-15-13-21(14-16-22)18-37-25-10-5-9-24(17-25)29-26-11-6-12-28(33(34,35)36)30(26)38-19-27(29)31(39)23-7-3-2-4-8-23/h2-17,19-20,37H,18H2,1H3,(H,40,41) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | 58 | n/a | n/a | n/a | n/a |
Wyeth Research
| Assay Description
Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... |
Bioorg Med Chem 15: 3321-33 (2007)
Article DOI: 10.1016/j.bmc.2007.03.013
BindingDB Entry DOI: 10.7270/Q2VH5M37 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-alpha
(Homo sapiens (Human)) | BDBM50306076

(1-(2-chloro-5-(3-(methylsulfonyl)phenoxy)phenyl)-4...)Show SMILES CS(=O)(=O)c1cccc(Oc2ccc(Cl)c(c2)-n2cnc3c(cccc23)C(F)(F)F)c1 Show InChI InChI=1S/C21H14ClF3N2O3S/c1-31(28,29)15-5-2-4-13(10-15)30-14-8-9-17(22)19(11-14)27-12-26-20-16(21(23,24)25)6-3-7-18(20)27/h2-12H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRalpha LBD |
Bioorg Med Chem Lett 20: 526-30 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.099
BindingDB Entry DOI: 10.7270/Q2HT2PDZ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta [154-461]
(Homo sapiens (Human)) | BDBM20014

(2-[4-({[3-(3-benzoyl-8-chloroquinolin-4-yl)phenyl]...)Show SMILES OC(=O)Cc1ccc(CNc2cccc(c2)-c2c(cnc3c(Cl)cccc23)C(=O)c2ccccc2)cc1 Show InChI InChI=1S/C31H23ClN2O3/c32-27-11-5-10-25-29(26(19-34-30(25)27)31(37)22-6-2-1-3-7-22)23-8-4-9-24(17-23)33-18-21-14-12-20(13-15-21)16-28(35)36/h1-15,17,19,33H,16,18H2,(H,35,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | 39 | n/a | n/a | n/a | n/a |
Wyeth Research
| Assay Description
Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... |
Bioorg Med Chem 15: 3321-33 (2007)
Article DOI: 10.1016/j.bmc.2007.03.013
BindingDB Entry DOI: 10.7270/Q2VH5M37 |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50317742
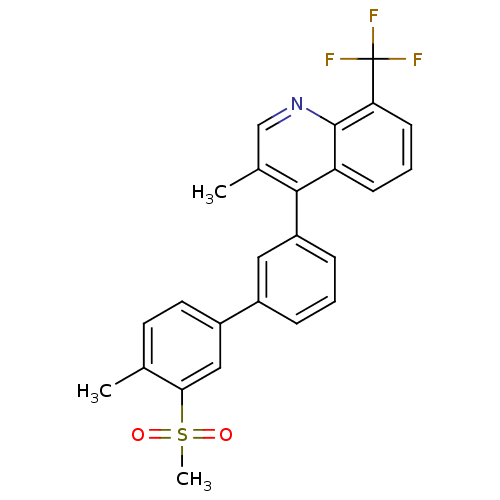
(3-methyl-4-(4'-methyl-3'-(methylsulfonyl)biphenyl-...)Show SMILES Cc1ccc(cc1S(C)(=O)=O)-c1cccc(c1)-c1c(C)cnc2c(cccc12)C(F)(F)F Show InChI InChI=1S/C25H20F3NO2S/c1-15-10-11-18(13-22(15)32(3,30)31)17-6-4-7-19(12-17)23-16(2)14-29-24-20(23)8-5-9-21(24)25(26,27)28/h4-14H,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from LXRbeta ligand binding domain |
Bioorg Med Chem Lett 20: 2903-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.031
BindingDB Entry DOI: 10.7270/Q2TD9XJZ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50306072

(2-isobutyl-1-(3-(3-(methylsulfonyl)phenoxy)phenyl)...)Show SMILES CC(C)Cc1nc2c(cccc2n1-c1cccc(Oc2cccc(c2)S(C)(=O)=O)c1)C(F)(F)F Show InChI InChI=1S/C25H23F3N2O3S/c1-16(2)13-23-29-24-21(25(26,27)28)11-6-12-22(24)30(23)17-7-4-8-18(14-17)33-19-9-5-10-20(15-19)34(3,31)32/h4-12,14-16H,13H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from human recombinant LXRbeta LBD |
Bioorg Med Chem Lett 20: 526-30 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.099
BindingDB Entry DOI: 10.7270/Q2HT2PDZ |
More data for this
Ligand-Target Pair | |
Oxysterols receptor LXR-beta
(Homo sapiens (Human)) | BDBM50317745
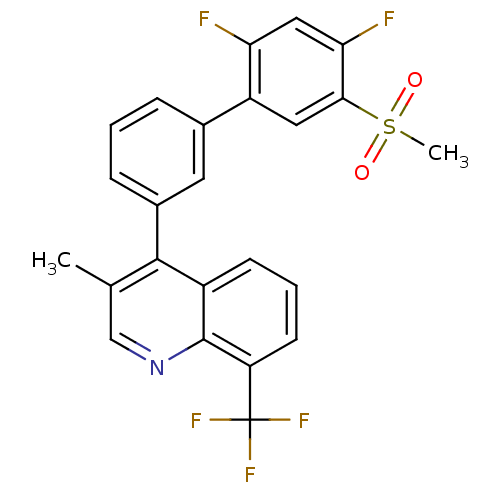
(4-(2',4'-difluoro-5'-(methylsulfonyl)biphenyl-3-yl...)Show SMILES Cc1cnc2c(cccc2c1-c1cccc(c1)-c1cc(c(F)cc1F)S(C)(=O)=O)C(F)(F)F Show InChI InChI=1S/C24H16F5NO2S/c1-13-12-30-23-16(7-4-8-18(23)24(27,28)29)22(13)15-6-3-5-14(9-15)17-10-21(33(2,31)32)20(26)11-19(17)25/h3-12H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]T0901317 from LXRbeta ligand binding domain |
Bioorg Med Chem Lett 20: 2903-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.031
BindingDB Entry DOI: 10.7270/Q2TD9XJZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data