

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50067536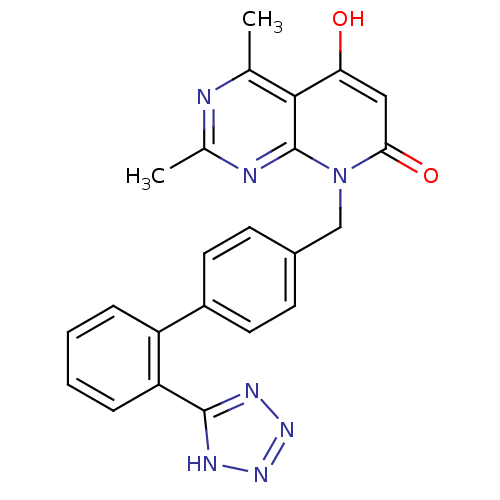 (5-Hydroxy-2,4-dimethyl-8-[2'-(1H-tetrazol-5-yl)-bi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Compound was evaluated for the in vitro inhibition of specific binding of [125I]-A II to Angiotensin II receptor, type 1 from rat adrenal membranes. | J Med Chem 41: 4251-60 (1998) Article DOI: 10.1021/jm970690q BindingDB Entry DOI: 10.7270/Q2FB55NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50152609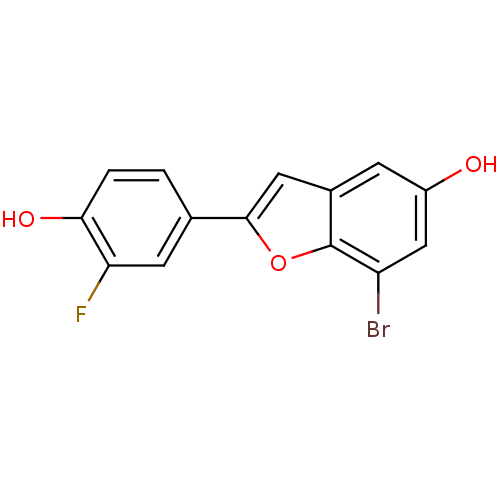 (7-Bromo-2-(3-fluoro-4-hydroxy-phenyl)-benzofuran-5...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Binding affinity for human Estrogen receptor beta | Bioorg Med Chem Lett 14: 4925-9 (2004) Article DOI: 10.1016/j.bmcl.2004.07.029 BindingDB Entry DOI: 10.7270/Q2TM79K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50471877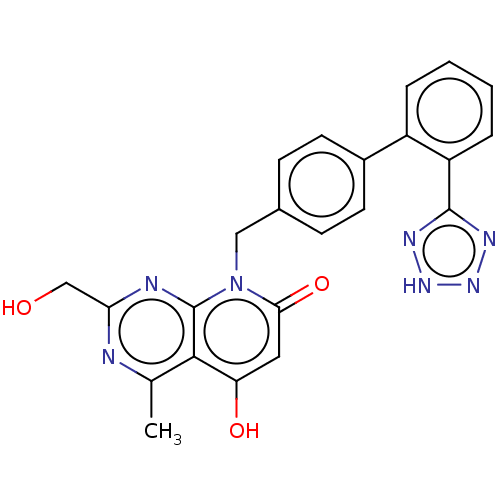 (CHEMBL135574) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Compound was evaluated for the in vitro inhibition of specific binding of [125I]-A II to Angiotensin II receptor, type 1 from rat adrenal membranes. | J Med Chem 41: 4251-60 (1998) Article DOI: 10.1021/jm970690q BindingDB Entry DOI: 10.7270/Q2FB55NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50152616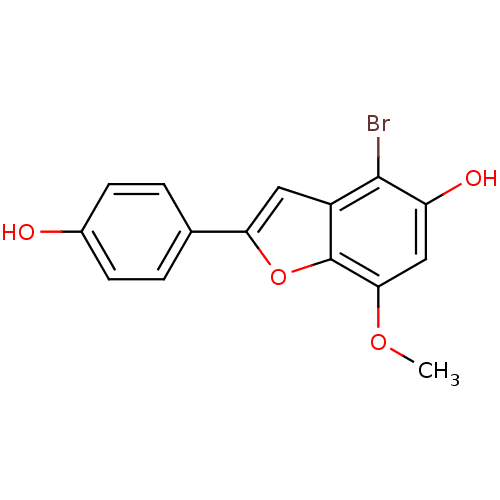 (4-Bromo-2-(4-hydroxy-phenyl)-7-methoxy-benzofuran-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Binding affinity for human Estrogen receptor beta | Bioorg Med Chem Lett 14: 4925-9 (2004) Article DOI: 10.1016/j.bmcl.2004.07.029 BindingDB Entry DOI: 10.7270/Q2TM79K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM19441 (2-(4-hydroxyphenyl)-3-({4-[2-(piperidin-1-yl)ethox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro antagonist effect on estrogen receptor alpha transcriptional activation in MCF-7 cells against 10 pM 17-beta-estradiol | J Med Chem 44: 1654-7 (2001) BindingDB Entry DOI: 10.7270/Q2F47NDX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50305077 (3-methyl-4-(3-(3-(methylsulfonyl)phenoxy)phenyl)-8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]TO901317 from human recombinant LXRbeta expressed in Escherichia coli by flashplate method | Bioorg Med Chem Lett 20: 689-93 (2010) Article DOI: 10.1016/j.bmcl.2009.11.062 BindingDB Entry DOI: 10.7270/Q2W66KV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50152628 (2-(3-Fluoro-4-hydroxy-phenyl)-5-hydroxy-benzofuran...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Binding affinity for human Estrogen receptor beta | Bioorg Med Chem Lett 14: 4925-9 (2004) Article DOI: 10.1016/j.bmcl.2004.07.029 BindingDB Entry DOI: 10.7270/Q2TM79K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Rattus norvegicus) | BDBM50154088 (7-Bromo-2-(4-hydroxy-phenyl)-benzooxazol-5-ol | 7-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of [3H]17-beta-estradiol binding to rat ER beta expressed in Escherichia coli | J Med Chem 47: 5021-40 (2004) Article DOI: 10.1021/jm049719y BindingDB Entry DOI: 10.7270/Q2C828S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50040439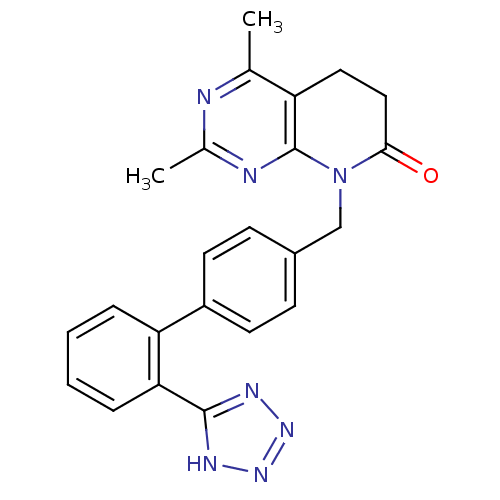 (2,4-Dimethyl-8-[2'-(1H-tetrazol-5-yl)-biphenyl-4-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Compound was evaluated for the in vitro inhibition of specific binding of [125I]-A II to Angiotensin II receptor, type 1 from rat adrenal membranes. | J Med Chem 41: 4251-60 (1998) Article DOI: 10.1021/jm970690q BindingDB Entry DOI: 10.7270/Q2FB55NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470071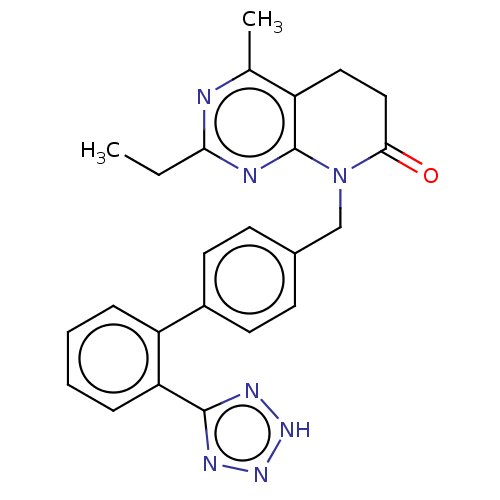 (CHEMBL150507) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of specific binding of [125 I] A II to rat liver membrane. | J Med Chem 37: 542-50 (1994) Article DOI: 10.1021/jm00030a013 BindingDB Entry DOI: 10.7270/Q2R78HX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50154078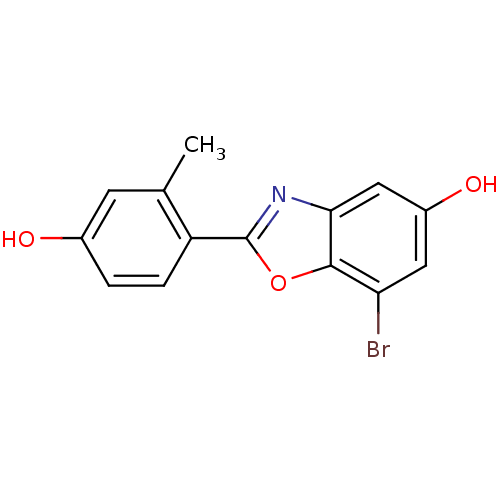 (7-Bromo-2-(4-hydroxy-2-methyl-phenyl)-benzooxazol-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibitory concentration against human ER beta expressed in Escherichia coli was determined using [3H]17-beta-estradiol as radio ligand | J Med Chem 47: 5021-40 (2004) Article DOI: 10.1021/jm049719y BindingDB Entry DOI: 10.7270/Q2C828S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470073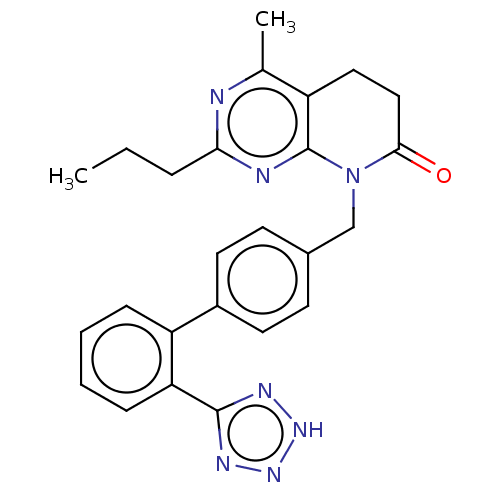 (CHEMBL153188) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of specific binding of [125 I] A II to rat liver membrane. | J Med Chem 37: 542-50 (1994) Article DOI: 10.1021/jm00030a013 BindingDB Entry DOI: 10.7270/Q2R78HX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50154059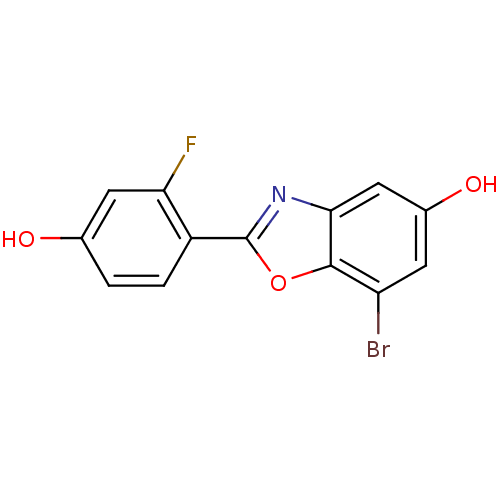 (7-Bromo-2-(2-fluoro-4-hydroxy-phenyl)-benzooxazol-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibitory concentration against human ER beta expressed in Escherichia coli was determined using [3H]17-beta-estradiol as radio ligand | J Med Chem 47: 5021-40 (2004) Article DOI: 10.1021/jm049719y BindingDB Entry DOI: 10.7270/Q2C828S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50154137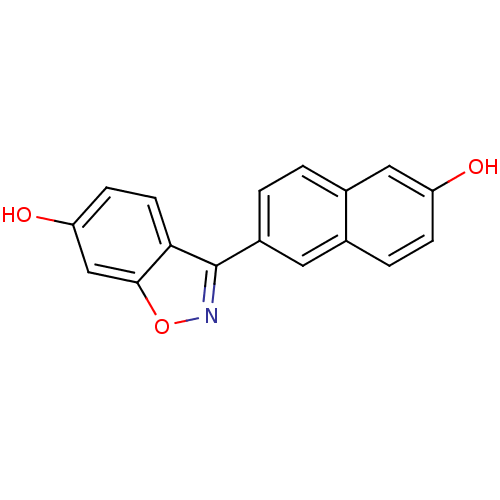 (3-(6-HYDROXY-NAPHTHALEN-2-YL)-BENZO[D]ISOOXAZOL-6-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibitory concentration against human ER beta expressed in Escherichia coli was determined using [3H]17-beta-estradiol as radio ligand | J Med Chem 47: 5021-40 (2004) Article DOI: 10.1021/jm049719y BindingDB Entry DOI: 10.7270/Q2C828S5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50154134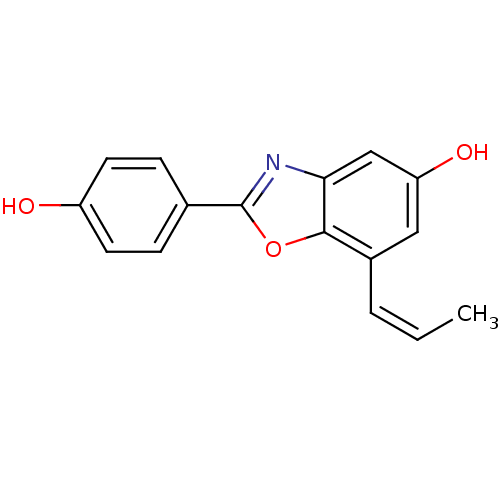 (2-(4-Hydroxy-phenyl)-7-propenyl-benzooxazol-5-ol |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibitory concentration against human ER beta expressed in Escherichia coli was determined using [3H]17-beta-estradiol as radio ligand | J Med Chem 47: 5021-40 (2004) Article DOI: 10.1021/jm049719y BindingDB Entry DOI: 10.7270/Q2C828S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50154048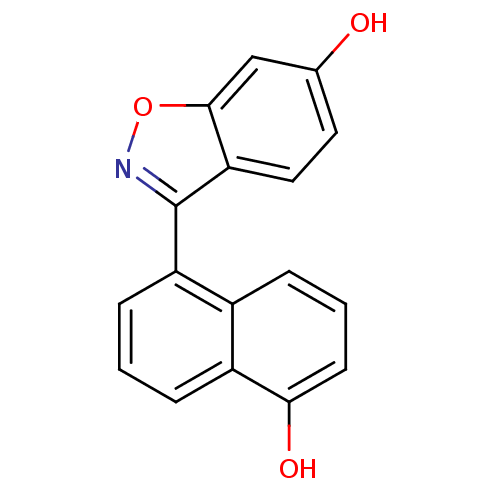 (3-(5-Hydroxy-naphthalen-1-yl)-benzo[d]isoxazol-6-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibitory concentration against human ER beta expressed in Escherichia coli was determined using [3H]17-beta-estradiol as radio ligand | J Med Chem 47: 5021-40 (2004) Article DOI: 10.1021/jm049719y BindingDB Entry DOI: 10.7270/Q2C828S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50099587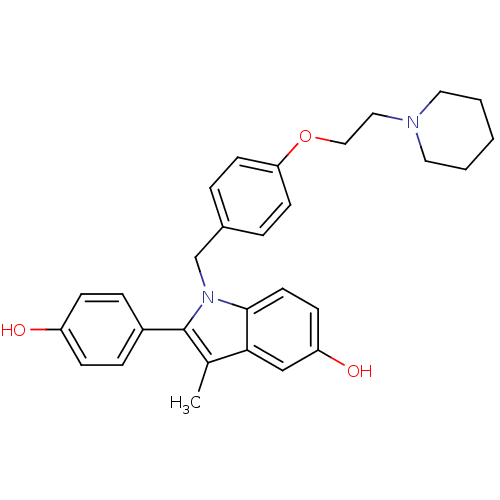 (2-(4-Hydroxy-phenyl)-3-methyl-1-[4-(2-piperidin-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro antagonist effect on estrogen receptor alpha transcriptional activation in MCF-7 cells against 10 pM 17-beta-estradiol | J Med Chem 44: 1654-7 (2001) BindingDB Entry DOI: 10.7270/Q2F47NDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50152629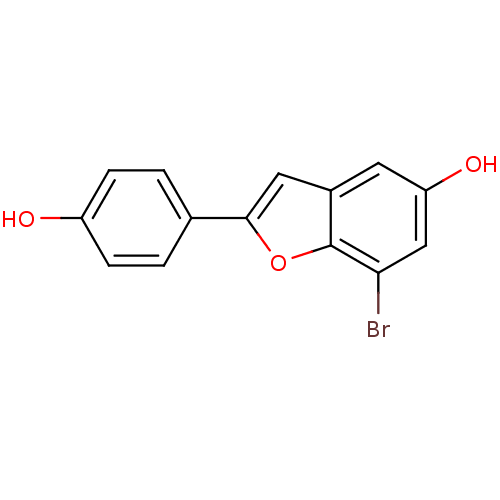 (7-Bromo-2-(4-hydroxy-phenyl)-benzofuran-5-ol | CHE...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Binding affinity for human Estrogen receptor alpha | Bioorg Med Chem Lett 14: 4925-9 (2004) Article DOI: 10.1016/j.bmcl.2004.07.029 BindingDB Entry DOI: 10.7270/Q2TM79K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50152626 (7-Chloro-2-(4-hydroxy-phenyl)-benzofuran-5-ol | CH...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Binding affinity for human Estrogen receptor beta | Bioorg Med Chem Lett 14: 4925-9 (2004) Article DOI: 10.1016/j.bmcl.2004.07.029 BindingDB Entry DOI: 10.7270/Q2TM79K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50130177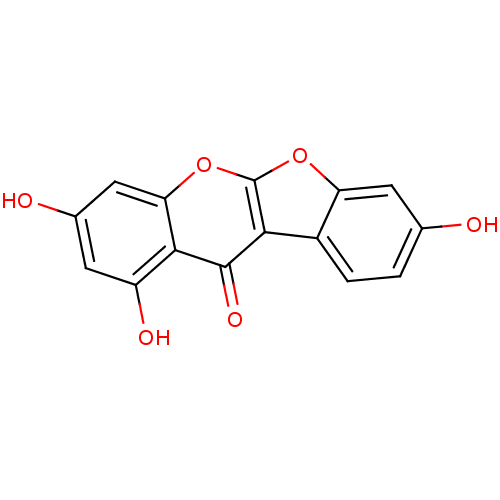 (2,6,8-Trihydroxy-10,11-dioxa-benzo[b]fluoren-5-one...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Binding affinity towards human estrogen receptor beta (ERbeta) | Bioorg Med Chem Lett 13: 2399-403 (2003) BindingDB Entry DOI: 10.7270/Q2TB169D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Rattus norvegicus) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of [3H]17-beta-estradiol binding to rat ER beta expressed in Escherichia coli | J Med Chem 47: 5021-40 (2004) Article DOI: 10.1021/jm049719y BindingDB Entry DOI: 10.7270/Q2C828S5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Mus musculus) | BDBM50154088 (7-Bromo-2-(4-hydroxy-phenyl)-benzooxazol-5-ol | 7-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of [3H]17-beta-estradiol binding to mouse ER beta expressed in Escherichia coli | J Med Chem 47: 5021-40 (2004) Article DOI: 10.1021/jm049719y BindingDB Entry DOI: 10.7270/Q2C828S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM20731 (4-bromo-6-(6-hydroxy-1,2-benzoxazol-3-yl)benzene-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibitory concentration against human ER beta expressed in Escherichia coli was determined using [3H]17-beta-estradiol as radio ligand | J Med Chem 47: 5021-40 (2004) Article DOI: 10.1021/jm049719y BindingDB Entry DOI: 10.7270/Q2C828S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470063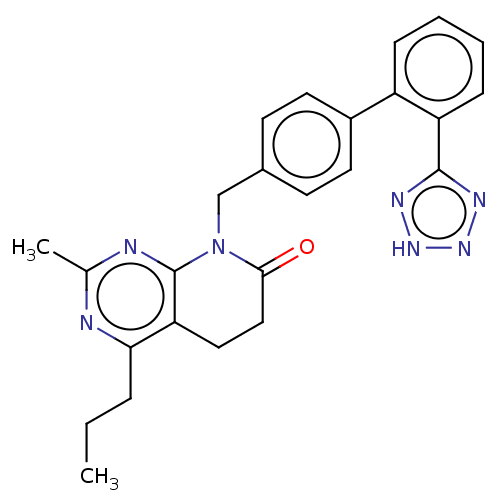 (CHEMBL152957) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of specific binding of [125 I] A II to rat liver membrane. | J Med Chem 37: 542-50 (1994) Article DOI: 10.1021/jm00030a013 BindingDB Entry DOI: 10.7270/Q2R78HX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM20001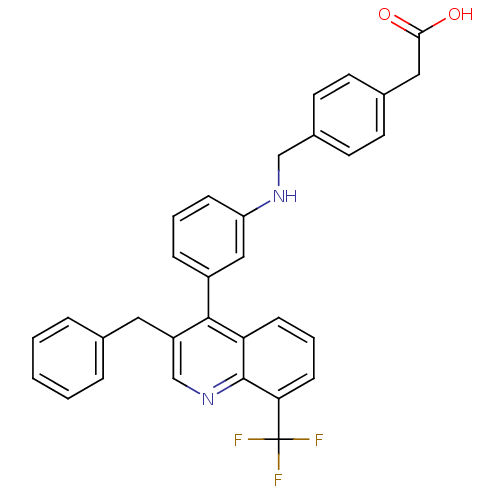 (2-{4-[({3-[3-benzyl-8-(trifluoromethyl)quinolin-4-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90 | n/a | 33 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | J Med Chem 49: 6151-4 (2006) Article DOI: 10.1021/jm0609566 BindingDB Entry DOI: 10.7270/Q20863KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (RAT) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of [3H]17-beta-estradiol binding to rat ER alpha expressed in Escherichia coli | J Med Chem 47: 5021-40 (2004) Article DOI: 10.1021/jm049719y BindingDB Entry DOI: 10.7270/Q2C828S5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50154140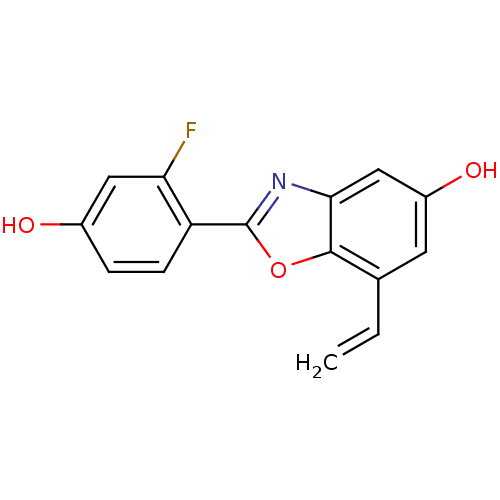 (2-(2-Fluoro-4-hydroxy-phenyl)-7-vinyl-benzooxazol-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibitory concentration against human ER beta expressed in Escherichia coli was determined using [3H]17-beta-estradiol as radio ligand | J Med Chem 47: 5021-40 (2004) Article DOI: 10.1021/jm049719y BindingDB Entry DOI: 10.7270/Q2C828S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50152629 (7-Bromo-2-(4-hydroxy-phenyl)-benzofuran-5-ol | CHE...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Binding affinity for human Estrogen receptor beta | Bioorg Med Chem Lett 14: 4925-9 (2004) Article DOI: 10.1016/j.bmcl.2004.07.029 BindingDB Entry DOI: 10.7270/Q2TM79K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50305501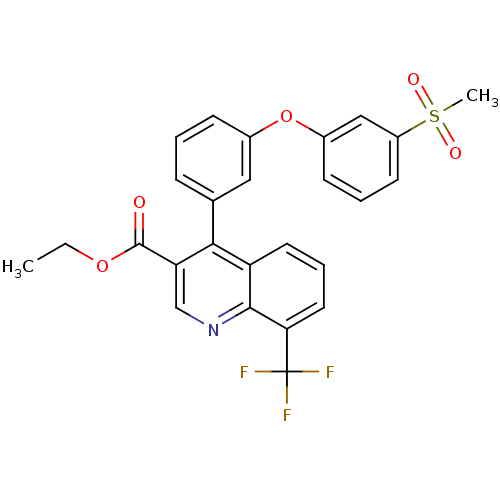 (CHEMBL590098 | ethyl 4-(3-(3-(methylsulfonyl)pheno...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]TO901317 from human recombinant LXRbeta expressed in Escherichia coli by flashplate method | Bioorg Med Chem Lett 20: 689-93 (2010) Article DOI: 10.1016/j.bmcl.2009.11.062 BindingDB Entry DOI: 10.7270/Q2W66KV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50227161 (2-(4-(3-(3-benzyl-8-(trifluoromethyl)quinolin-4-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity at human LXRbeta | Bioorg Med Chem Lett 18: 54-9 (2008) Article DOI: 10.1016/j.bmcl.2007.11.013 BindingDB Entry DOI: 10.7270/Q2N879JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50227146 (2-(4-(3-(3-benzyl-8-(trifluoromethyl)quinolin-4-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity at human LXRbeta | Bioorg Med Chem Lett 18: 54-9 (2008) Article DOI: 10.1016/j.bmcl.2007.11.013 BindingDB Entry DOI: 10.7270/Q2N879JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50227163 (2-(4-((3-(3-benzoyl-8-(trifluoromethyl)quinolin-5-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity at human LXRbeta | Bioorg Med Chem Lett 18: 54-9 (2008) Article DOI: 10.1016/j.bmcl.2007.11.013 BindingDB Entry DOI: 10.7270/Q2N879JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM20000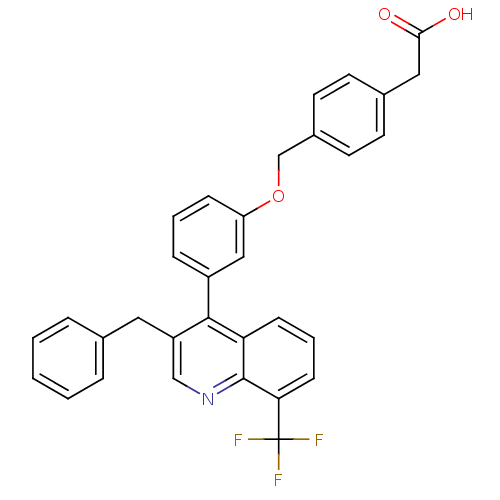 (2-(4-{3-[3-benzyl-8-(trifluoromethyl)quinolin-4-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity at human LXRbeta | Bioorg Med Chem Lett 18: 54-9 (2008) Article DOI: 10.1016/j.bmcl.2007.11.013 BindingDB Entry DOI: 10.7270/Q2N879JT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50154088 (7-Bromo-2-(4-hydroxy-phenyl)-benzooxazol-5-ol | 7-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibitory concentration against human ER beta expressed in Escherichia coli was determined using [3H]17-beta-estradiol as radio ligand | J Med Chem 47: 5021-40 (2004) Article DOI: 10.1021/jm049719y BindingDB Entry DOI: 10.7270/Q2C828S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50152617 (CHEMBL360385 | [4-Bromo-5-hydroxy-2-(4-hydroxy-phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Binding affinity for human Estrogen receptor beta | Bioorg Med Chem Lett 14: 4925-9 (2004) Article DOI: 10.1016/j.bmcl.2004.07.029 BindingDB Entry DOI: 10.7270/Q2TM79K8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Rattus norvegicus) | BDBM50154084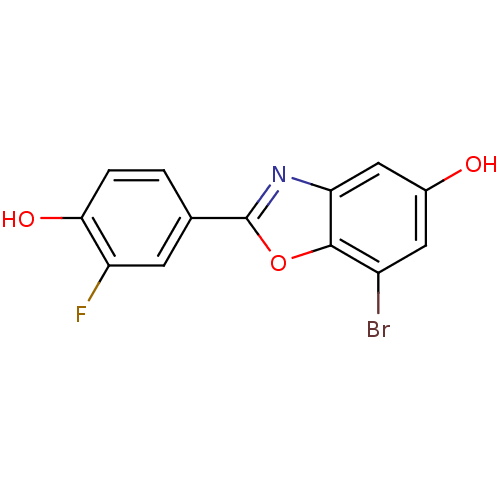 (7-Bromo-2-(3-fluoro-4-hydroxy-phenyl)-benzooxazol-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of [3H]17-beta-estradiol binding to rat ER beta expressed in Escherichia coli | J Med Chem 47: 5021-40 (2004) Article DOI: 10.1021/jm049719y BindingDB Entry DOI: 10.7270/Q2C828S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM20734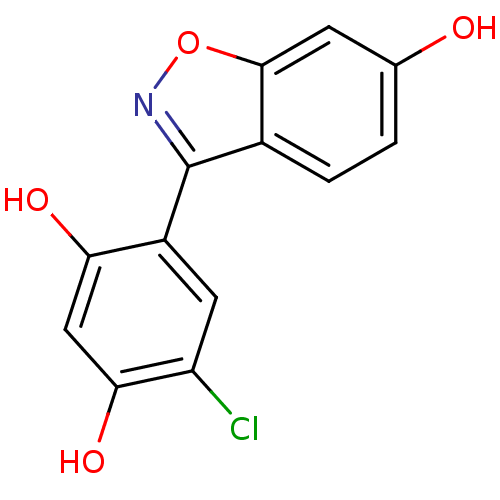 (4-chloro-6-(6-hydroxy-1,2-benzoxazol-3-yl)benzene-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibitory concentration against human ER beta expressed in Escherichia coli was determined using [3H]17-beta-estradiol as radio ligand | J Med Chem 47: 5021-40 (2004) Article DOI: 10.1021/jm049719y BindingDB Entry DOI: 10.7270/Q2C828S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50305077 (3-methyl-4-(3-(3-(methylsulfonyl)phenoxy)phenyl)-8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]TO901317 from human recombinant LXRalpha expressed in Escherichia coli by flashplate method | Bioorg Med Chem Lett 20: 689-93 (2010) Article DOI: 10.1016/j.bmcl.2009.11.062 BindingDB Entry DOI: 10.7270/Q2W66KV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM50227157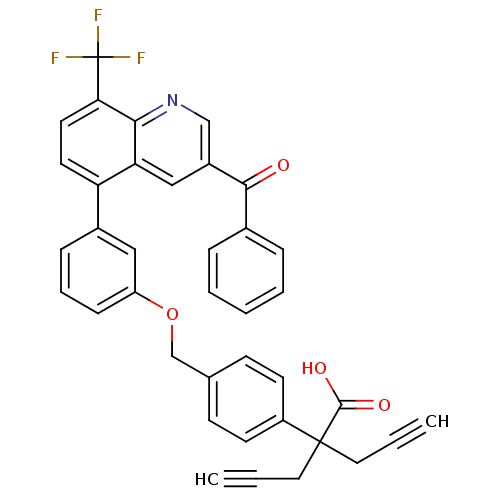 (2-(4-((3-(3-benzoyl-8-(trifluoromethyl)quinolin-5-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity at human LXRalpha | Bioorg Med Chem Lett 18: 54-9 (2008) Article DOI: 10.1016/j.bmcl.2007.11.013 BindingDB Entry DOI: 10.7270/Q2N879JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM20000 (2-(4-{3-[3-benzyl-8-(trifluoromethyl)quinolin-4-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.10 | n/a | 71 | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | J Med Chem 49: 6151-4 (2006) Article DOI: 10.1021/jm0609566 BindingDB Entry DOI: 10.7270/Q20863KH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50154132 (2-(2,6-Difluoro-4-hydroxy-phenyl)-7-vinyl-benzooxa...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibitory concentration against human ER beta expressed in Escherichia coli was determined using [3H]17-beta-estradiol as radio ligand | J Med Chem 47: 5021-40 (2004) Article DOI: 10.1021/jm049719y BindingDB Entry DOI: 10.7270/Q2C828S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50152624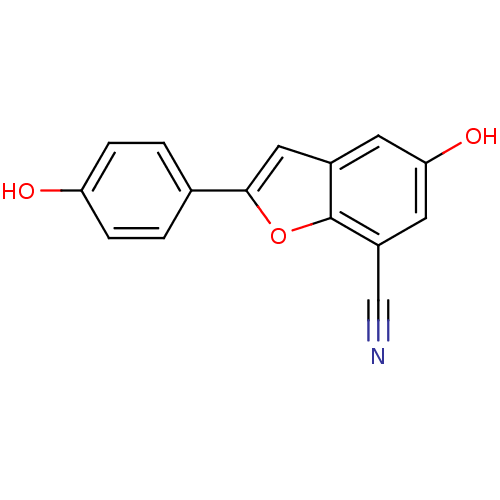 (5-HYDROXY-2-(4-HYDROXYPHENYL)-1-BENZOFURAN-7-CARBO...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Binding affinity for human Estrogen receptor beta | Bioorg Med Chem Lett 14: 4925-9 (2004) Article DOI: 10.1016/j.bmcl.2004.07.029 BindingDB Entry DOI: 10.7270/Q2TM79K8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470065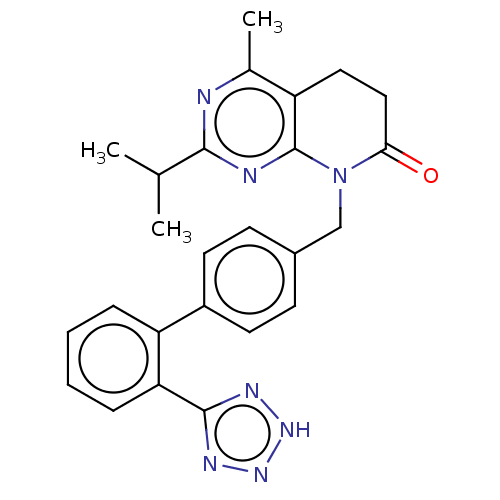 (CHEMBL348472) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of specific binding of [125 I] A II to rat liver membrane. | J Med Chem 37: 542-50 (1994) Article DOI: 10.1021/jm00030a013 BindingDB Entry DOI: 10.7270/Q2R78HX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM50305499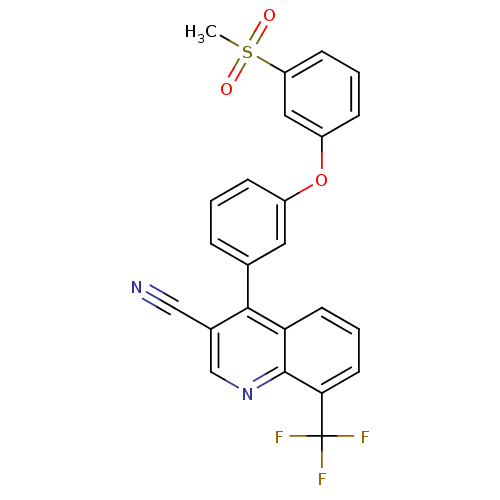 (4-(3-(3-(methylsulfonyl)phenoxy)phenyl)-8-(trifluo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]TO901317 from human recombinant LXRbeta expressed in Escherichia coli by flashplate method | Bioorg Med Chem Lett 20: 689-93 (2010) Article DOI: 10.1016/j.bmcl.2009.11.062 BindingDB Entry DOI: 10.7270/Q2W66KV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Rattus norvegicus) | BDBM50154057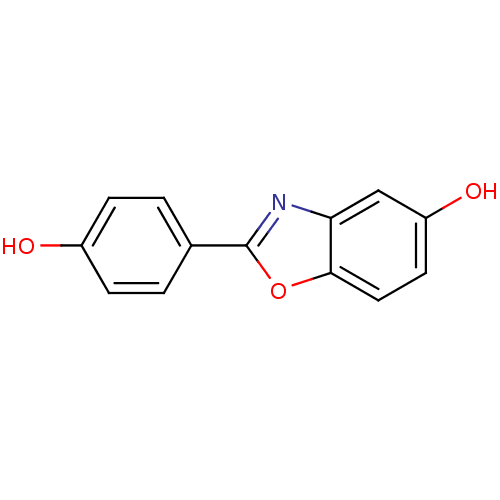 (2-(4-Hydroxy-phenyl)-benzooxazol-5-ol | 2-(4-hydro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of [3H]17-beta-estradiol binding to rat ER beta expressed in Escherichia coli | J Med Chem 47: 5021-40 (2004) Article DOI: 10.1021/jm049719y BindingDB Entry DOI: 10.7270/Q2C828S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Mus musculus) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of [3H]17-beta-estradiol binding to mouse ER alpha expressed in Escherichia coli | J Med Chem 47: 5021-40 (2004) Article DOI: 10.1021/jm049719y BindingDB Entry DOI: 10.7270/Q2C828S5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50471878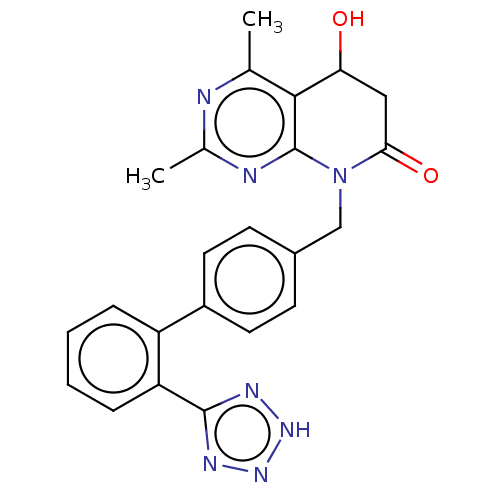 (CHEMBL337067) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Compound was evaluated for the in vitro inhibition of specific binding of [125I]-A II to Angiotensin II receptor, type 1 from rat adrenal membranes. | J Med Chem 41: 4251-60 (1998) Article DOI: 10.1021/jm970690q BindingDB Entry DOI: 10.7270/Q2FB55NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470068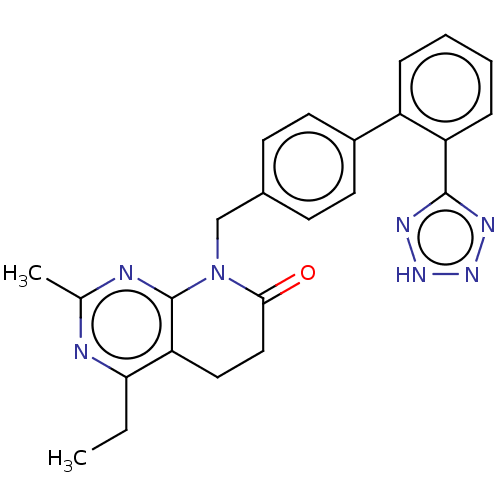 (CHEMBL152958) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of specific binding of [125 I] A II to rat liver membrane. | J Med Chem 37: 542-50 (1994) Article DOI: 10.1021/jm00030a013 BindingDB Entry DOI: 10.7270/Q2R78HX6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Mus musculus) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of [3H]17-beta-estradiol binding to mouse ER beta expressed in Escherichia coli | J Med Chem 47: 5021-40 (2004) Article DOI: 10.1021/jm049719y BindingDB Entry DOI: 10.7270/Q2C828S5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50154064 (2-(2-Fluoro-4-hydroxy-phenyl)-5-hydroxy-benzooxazo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibitory concentration against human ER beta expressed in Escherichia coli was determined using [3H]17-beta-estradiol as radio ligand | J Med Chem 47: 5021-40 (2004) Article DOI: 10.1021/jm049719y BindingDB Entry DOI: 10.7270/Q2C828S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 580 total ) | Next | Last >> |