

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50121414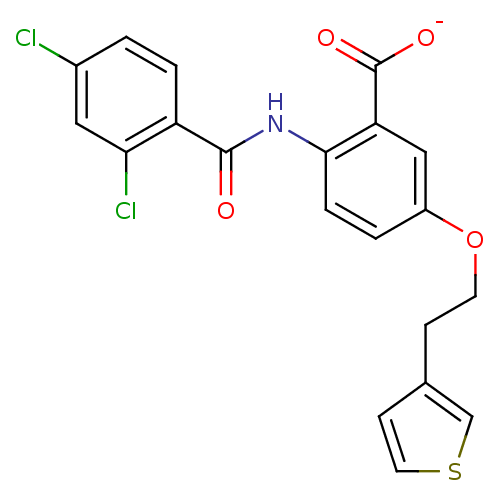 (CHEMBL333890 | Lithium; 2-(2,4-dichloro-benzoylami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Transactivation potency was measured by luciferase activity in Caco- 2/TC7 cells transiently co-transfected for the fusion-protein Gal4-PPAR alpha | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50121414 (CHEMBL333890 | Lithium; 2-(2,4-dichloro-benzoylami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Transactivation potency was measured by luciferase activity in Caco- 2/TC7 cells transiently co-transfected for the fusion-protein Gal4-PPAR alpha | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||