

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50136234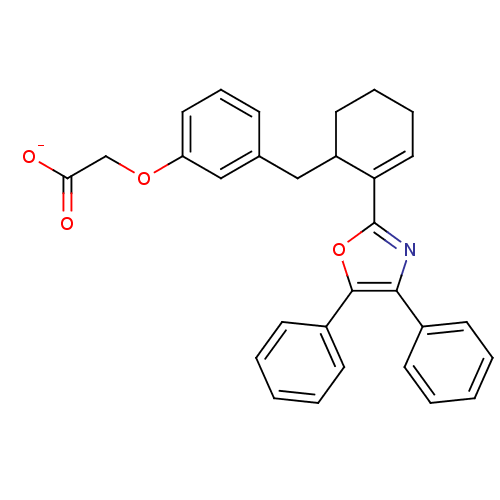 (CHEMBL132589 | FR-181157 | Sodium; {3-[(R)-2-(4,5-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co. Ltd Curated by ChEMBL | Assay Description In vitro Prostacyclin (PGI-2) receptor binding assay was determined based on displacement of [3H]-Iloprost radioligand from cloned human IP receptor | Bioorg Med Chem Lett 13: 4277-9 (2003) BindingDB Entry DOI: 10.7270/Q22R3R3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50136234 (CHEMBL132589 | FR-181157 | Sodium; {3-[(R)-2-(4,5-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-Iloprost binding to human prostanoid IP receptor | J Med Chem 48: 3103-6 (2005) Article DOI: 10.1021/jm050085k BindingDB Entry DOI: 10.7270/Q2XD116M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50136234 (CHEMBL132589 | FR-181157 | Sodium; {3-[(R)-2-(4,5-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of [3H]-Iloprost binding to human Prostanoid IP receptor | Bioorg Med Chem Lett 15: 3284-7 (2005) Article DOI: 10.1016/j.bmcl.2005.04.076 BindingDB Entry DOI: 10.7270/Q2C53KCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50136234 (CHEMBL132589 | FR-181157 | Sodium; {3-[(R)-2-(4,5-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]iloprost from cloned human PGI2 receptor | Bioorg Med Chem Lett 16: 4861-4 (2006) Article DOI: 10.1016/j.bmcl.2006.06.076 BindingDB Entry DOI: 10.7270/Q2R49QD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50136234 (CHEMBL132589 | FR-181157 | Sodium; {3-[(R)-2-(4,5-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Agonist activity at human PGI2 receptor assessed as inhibition of ADP-induced platelet aggregation | Bioorg Med Chem Lett 16: 4861-4 (2006) Article DOI: 10.1016/j.bmcl.2006.06.076 BindingDB Entry DOI: 10.7270/Q2R49QD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (RAT) | BDBM50136234 (CHEMBL132589 | FR-181157 | Sodium; {3-[(R)-2-(4,5-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Agonist activity at rat PGI2 receptor assessed as inhibition of ADP-induced platelet aggregation | Bioorg Med Chem Lett 16: 4861-4 (2006) Article DOI: 10.1016/j.bmcl.2006.06.076 BindingDB Entry DOI: 10.7270/Q2R49QD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||