

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin E2 receptor EP2 subtype (Mus musculus (Mouse)) | BDBM50101858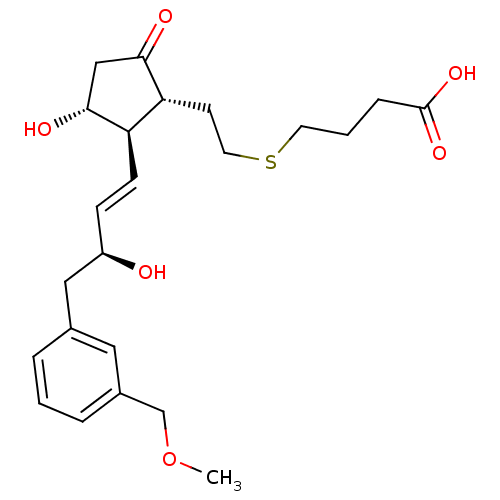 (4-(2-((1R,2R,3R)-3-hydroxy-2-((S,E)-3-hydroxy-4-(3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Affinity for mouse Prostanoid EP2 receptor expressed in CHO cells | Bioorg Med Chem Lett 11: 2033-5 (2001) BindingDB Entry DOI: 10.7270/Q24B30K2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Mus musculus (Mouse)) | BDBM50101858 (4-(2-((1R,2R,3R)-3-hydroxy-2-((S,E)-3-hydroxy-4-(3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Binding affinity to mouse EP2 receptor by competitive binding assay | J Med Chem 53: 4332-53 (2010) Article DOI: 10.1021/jm9018756 BindingDB Entry DOI: 10.7270/Q21R6QPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Mus musculus (Mouse)) | BDBM50101858 (4-(2-((1R,2R,3R)-3-hydroxy-2-((S,E)-3-hydroxy-4-(3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]-PGE2 from mouse EP2 receptor expressed in CHO cells after 60 mins by liquid scintillation counter | Bioorg Med Chem 20: 702-13 (2012) Checked by Author Article DOI: 10.1016/j.bmc.2011.12.009 BindingDB Entry DOI: 10.7270/Q2CJ8DXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM50101858 (4-(2-((1R,2R,3R)-3-hydroxy-2-((S,E)-3-hydroxy-4-(3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 610 | n/a | n/a | n/a | n/a |
Cayman Chemical Company, Inc. Curated by ChEMBL | Assay Description Agonist activity at human EP2 receptor expressed in HEK293T/17 cells assessed as increase in GalphaS-mediated CREB activation measured after 6 to 24 ... | J Med Chem 62: 4731-4741 (2019) Article DOI: 10.1021/acs.jmedchem.9b00336 BindingDB Entry DOI: 10.7270/Q22R3W32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM50101858 (4-(2-((1R,2R,3R)-3-hydroxy-2-((S,E)-3-hydroxy-4-(3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a |
Cayman Chemical Company, Inc. Curated by ChEMBL | Assay Description Agonist activity at human EP2 receptor expressed in HEK293T/17 cells assessed as increase in intracellular cAMP level incubated for 30 mins by ELISA | J Med Chem 62: 4731-4741 (2019) Article DOI: 10.1021/acs.jmedchem.9b00336 BindingDB Entry DOI: 10.7270/Q22R3W32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||