

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50227935 (CHEMBL75708) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Acyl coenzyme A:cholesterol acyltransferase derived from rat adrenals | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50227908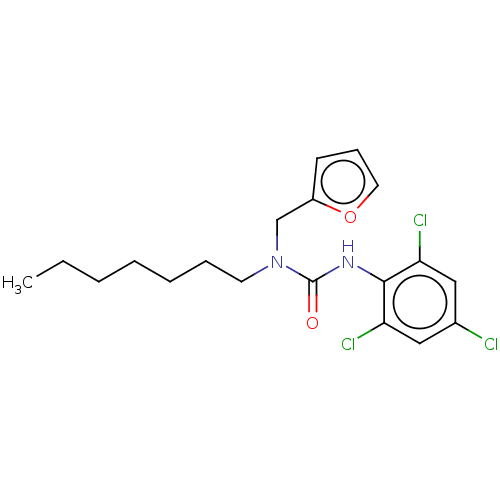 (CHEMBL77988) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 406 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Acyl coenzyme A:cholesterol acyltransferase derived from rat adrenals | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50227918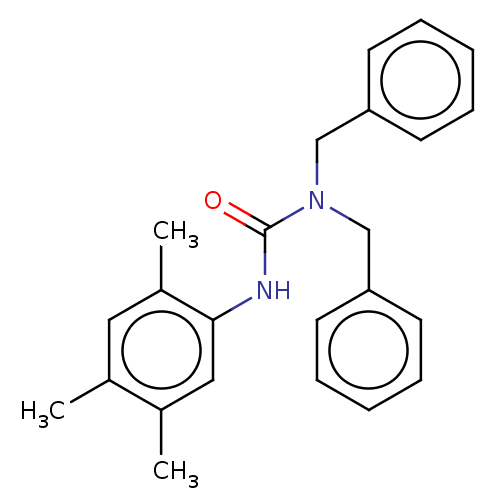 (CHEMBL75224) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 446 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Acyl coenzyme A:cholesterol acyltransferase derived from rat adrenals | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50227912 (CHEMBL75204) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 484 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Acyl coenzyme A:cholesterol acyltransferase derived from rat adrenals | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50227931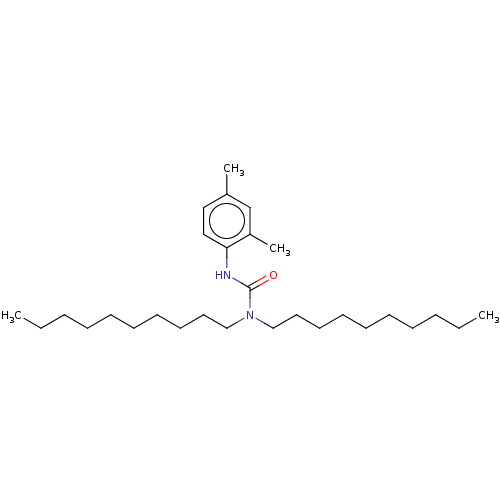 (CHEMBL76938) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 629 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Acyl coenzyme A:cholesterol acyltransferase derived from rat adrenals | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50227921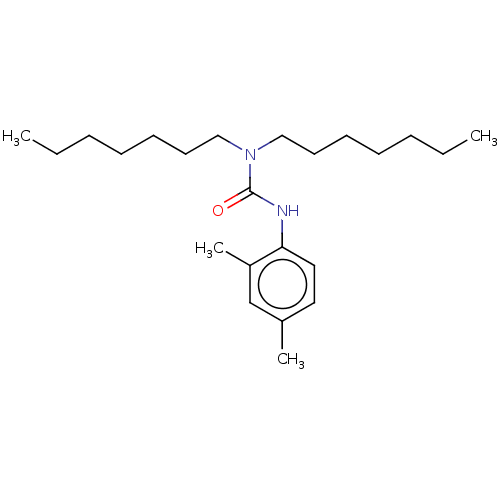 (CHEMBL75709) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 721 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Acyl coenzyme A:cholesterol acyltransferase derived from rat adrenals | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50227932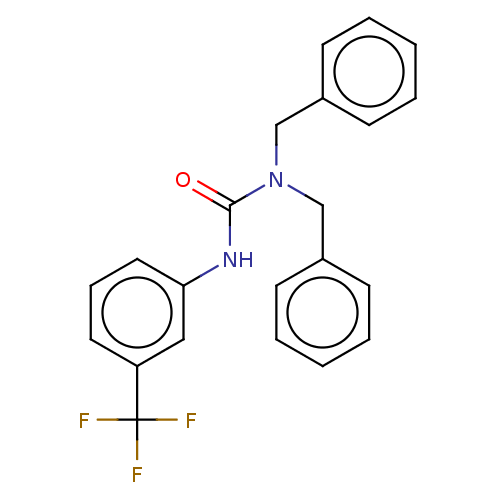 (CHEMBL77164) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Acyl coenzyme A:cholesterol acyltransferase derived from rat adrenals | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50227944 (CHEMBL77942) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Acyl coenzyme A:cholesterol acyltransferase derived from rat adrenals | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50227934 (CHEMBL75371) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Acyl coenzyme A:cholesterol acyltransferase derived from rat adrenals | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50227917 (CHEMBL77694) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Acyl coenzyme A:cholesterol acyltransferase derived from rat adrenals | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50227942 (CHEMBL76016) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Acyl coenzyme A:cholesterol acyltransferase derived from rat adrenals | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50227904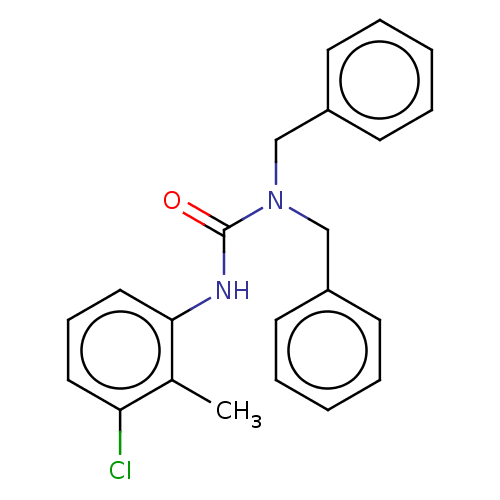 (CHEMBL74961) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Acyl coenzyme A:cholesterol acyltransferase derived from rat adrenals | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Rattus norvegicus) | BDBM50227937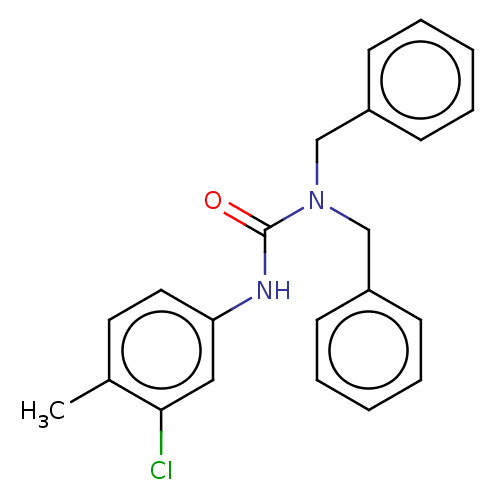 (CHEMBL76258) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 1.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
American Cyanamid Company Curated by ChEMBL | Assay Description In vitro inhibitory concentration against Acyl coenzyme A:cholesterol acyltransferase derived from rat adrenals | J Med Chem 32: 2318-25 (1989) BindingDB Entry DOI: 10.7270/Q2QZ2D5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||