 Found 32 hits of ec50 for UniProtKB: P32238
Found 32 hits of ec50 for UniProtKB: P32238 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.00900 | n/a | n/a | n/a | n/a |
Mayo Clinic and Foundation
Curated by ChEMBL
| Assay Description
Ability to stimulate intracellular calcium response was determined using CCK receptor-bearing chinese hamster ovary cell line |
J Med Chem 42: 2105-11 (1999)
Article DOI: 10.1021/jm980732q
BindingDB Entry DOI: 10.7270/Q27P924Q |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50004477
(CCK7 analogue | CHEMBL269016 | N-(1-Carbamoyl-2-ph...)Show SMILES CCCC[C@H](NC(=O)CCc1ccc(OS(O)(=O)=O)cc1)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCC)C(=O)N(C)[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C48H62N8O13S/c1-4-6-16-36(52-41(57)24-21-30-19-22-33(23-20-30)69-70(66,67)68)45(62)51-29-42(58)53-39(26-32-28-50-35-18-12-11-15-34(32)35)46(63)54-37(17-7-5-2)48(65)56(3)40(27-43(59)60)47(64)55-38(44(49)61)25-31-13-9-8-10-14-31/h8-15,18-20,22-23,28,36-40,50H,4-7,16-17,21,24-27,29H2,1-3H3,(H2,49,61)(H,51,62)(H,52,57)(H,53,58)(H,54,63)(H,55,64)(H,59,60)(H,66,67,68)/t36-,37-,38-,39-,40-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Thermodynamic dissociation constant of compound for wild type E. coli dihydrofolate reductase |
J Med Chem 35: 2919-28 (1992)
BindingDB Entry DOI: 10.7270/Q2W66JRH |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50004482
(CCK7 analogue | CHEMBL263742 | N-(1-Carbamoyl-2-ph...)Show SMILES CCCC[C@H](NC(=O)CCc1ccc(OS(O)(=O)=O)cc1)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCC)C(=O)N[C@@H](CC(O)=O)C(=O)N(C)[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C48H62N8O13S/c1-4-6-16-36(52-41(57)24-21-30-19-22-33(23-20-30)69-70(66,67)68)45(62)51-29-42(58)53-38(26-32-28-50-35-18-12-11-15-34(32)35)47(64)54-37(17-7-5-2)46(63)55-39(27-43(59)60)48(65)56(3)40(44(49)61)25-31-13-9-8-10-14-31/h8-15,18-20,22-23,28,36-40,50H,4-7,16-17,21,24-27,29H2,1-3H3,(H2,49,61)(H,51,62)(H,52,57)(H,53,58)(H,54,64)(H,55,63)(H,59,60)(H,66,67,68)/t36-,37-,38-,39-,40-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Thermodynamic dissociation constant of compound for mutant T46A E. coli dihydrofolate reductase |
J Med Chem 35: 2919-28 (1992)
BindingDB Entry DOI: 10.7270/Q2W66JRH |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50004436

(3-Acetylamino-N-[1-[1-({[1-{1-[1-(1-carbamoyl-2-ph...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CN(C)C(=O)[C@H](CCCC)NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@H](CC(O)=O)NC(C)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C54H70N10O17S/c1-5-7-17-38(49(72)63-44(28-47(69)70)53(76)61-40(48(55)71)24-32-14-10-9-11-15-32)59-51(74)42(26-34-29-56-37-19-13-12-16-36(34)37)58-45(66)30-64(4)54(77)39(18-8-6-2)60-50(73)41(62-52(75)43(27-46(67)68)57-31(3)65)25-33-20-22-35(23-21-33)81-82(78,79)80/h9-16,19-23,29,38-44,56H,5-8,17-18,24-28,30H2,1-4H3,(H2,55,71)(H,57,65)(H,58,66)(H,59,74)(H,60,73)(H,61,76)(H,62,75)(H,63,72)(H,67,68)(H,69,70)(H,78,79,80)/t38-,39-,40-,41-,42-,43-,44-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a |
Hadassah-University Hospital
Curated by ChEMBL
| Assay Description
In vitro smooth muscle contraction activity in guinea pig ileum consists of two or three CCK receptor subtypes |
J Med Chem 35: 2806-11 (1992)
BindingDB Entry DOI: 10.7270/Q28C9V6B |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50004480
(CCK7 analogue | CHEMBL316944)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)CCc1ccc(OS(O)(=O)=O)cc1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C47H60N8O13S/c1-4-5-14-35(45(62)55-39(25-42(58)59)47(64)54-36(43(48)60)23-30-11-7-6-8-12-30)53-46(63)38(24-31-26-49-34-15-10-9-13-33(31)34)52-41(57)27-50-44(61)37(22-28(2)3)51-40(56)21-18-29-16-19-32(20-17-29)68-69(65,66)67/h6-13,15-17,19-20,26,28,35-39,49H,4-5,14,18,21-25,27H2,1-3H3,(H2,48,60)(H,50,61)(H,51,56)(H,52,57)(H,53,63)(H,54,64)(H,55,62)(H,58,59)(H,65,66,67)/t35-,36-,37-,38-,39-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 0.640 | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Dissociation rate constant of compound for mutant T46A Escherichia coli dihydrofolate reductase |
J Med Chem 35: 2919-28 (1992)
BindingDB Entry DOI: 10.7270/Q2W66JRH |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50421963
(CHEMBL2310856)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CNC(=O)[C@@H]1CCCN1C(=O)CCc1ccc(OS(O)(=O)=O)cc1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C46H56N8O13S/c1-2-3-13-34(43(60)53-37(25-41(57)58)45(62)52-35(42(47)59)23-29-10-5-4-6-11-29)51-44(61)36(24-30-26-48-33-14-8-7-12-32(30)33)50-39(55)27-49-46(63)38-15-9-22-54(38)40(56)21-18-28-16-19-31(20-17-28)67-68(64,65)66/h4-8,10-12,14,16-17,19-20,26,34-38,48H,2-3,9,13,15,18,21-25,27H2,1H3,(H2,47,59)(H,49,63)(H,50,55)(H,51,61)(H,52,62)(H,53,60)(H,57,58)(H,64,65,66)/t34-,35-,36-,37-,38-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitor constant of compound for wild type E. coli dihydrofolate reductase |
J Med Chem 35: 2919-28 (1992)
BindingDB Entry DOI: 10.7270/Q2W66JRH |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50004437
(3-Acetylamino-N-[1-[1-({[1-(1-{[1-(1-carbamoyl-2-p...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@H](CC(O)=O)NC(C)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCC)C(=O)N(C)[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C54H70N10O17S/c1-5-7-17-38(60-50(73)41(63-52(75)43(27-46(67)68)58-31(3)65)25-33-20-22-35(23-21-33)81-82(78,79)80)49(72)57-30-45(66)59-42(26-34-29-56-37-19-13-12-16-36(34)37)51(74)61-39(18-8-6-2)54(77)64(4)44(28-47(69)70)53(76)62-40(48(55)71)24-32-14-10-9-11-15-32/h9-16,19-23,29,38-44,56H,5-8,17-18,24-28,30H2,1-4H3,(H2,55,71)(H,57,72)(H,58,65)(H,59,66)(H,60,73)(H,61,74)(H,62,76)(H,63,75)(H,67,68)(H,69,70)(H,78,79,80)/t38-,39-,40-,41-,42-,43-,44-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a |
Hadassah-University Hospital
Curated by ChEMBL
| Assay Description
In vitro smooth muscle contraction activity in guinea pig ileum consists of two or three CCK receptor subtypes |
J Med Chem 35: 2806-11 (1992)
BindingDB Entry DOI: 10.7270/Q28C9V6B |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50004474

(CCK7 analogue | CHEMBL316949 | N-(1-Carbamoyl-2-ph...)Show SMILES CCCC[C@H](NC(=O)CCc1ccc(OS(O)(=O)=O)cc1)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C47H60N8O13S/c1-3-5-15-35(51-40(56)23-20-29-18-21-32(22-19-29)68-69(65,66)67)44(61)50-28-41(57)52-38(25-31-27-49-34-17-11-10-14-33(31)34)46(63)53-36(16-6-4-2)45(62)55-39(26-42(58)59)47(64)54-37(43(48)60)24-30-12-8-7-9-13-30/h7-14,17-19,21-22,27,35-39,49H,3-6,15-16,20,23-26,28H2,1-2H3,(H2,48,60)(H,50,61)(H,51,56)(H,52,57)(H,53,63)(H,54,64)(H,55,62)(H,58,59)(H,65,66,67)/t35-,36-,37-,38-,39-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 0.990 | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Dissociation rate constant of compound for mutant T46A Escherichia coli dihydrofolate reductase |
J Med Chem 35: 2919-28 (1992)
BindingDB Entry DOI: 10.7270/Q2W66JRH |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50004483

(CCK7 analogue | CHEMBL269355)Show SMILES CCCC[C@H](NC(=O)CCc1ccc(OS(O)(=O)=O)cc1)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N1CCC(CCC)[C@H]1C(=O)N[C@@H](CC(O)=O)C(=O)N(C)[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C50H64N8O13S/c1-4-6-16-38(54-42(59)23-20-31-18-21-35(22-19-31)71-72(68,69)70)47(64)53-30-43(60)55-39(27-34-29-52-37-17-11-10-15-36(34)37)50(67)58-25-24-33(12-5-2)45(58)48(65)56-40(28-44(61)62)49(66)57(3)41(46(51)63)26-32-13-8-7-9-14-32/h7-11,13-15,17-19,21-22,29,33,38-41,45,52H,4-6,12,16,20,23-28,30H2,1-3H3,(H2,51,63)(H,53,64)(H,54,59)(H,55,60)(H,56,65)(H,61,62)(H,68,69,70)/t33?,38-,39-,40-,41-,45-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Dissociation rate constant of compound for wild type Escherichia coli dihydrofolate reductase |
J Med Chem 35: 2919-28 (1992)
BindingDB Entry DOI: 10.7270/Q2W66JRH |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50004440
(3-Acetylamino-N-[1-[1-({[1-{1-[1-(1-carbamoyl-2-ph...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@H](CC(O)=O)NC(C)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C53H68N10O17S/c1-4-6-16-37(59-50(73)40(62-52(75)42(26-45(66)67)57-30(3)64)24-32-19-21-34(22-20-32)80-81(77,78)79)48(71)56-29-44(65)58-41(25-33-28-55-36-18-12-11-15-35(33)36)51(74)60-38(17-7-5-2)49(72)63-43(27-46(68)69)53(76)61-39(47(54)70)23-31-13-9-8-10-14-31/h8-15,18-22,28,37-43,55H,4-7,16-17,23-27,29H2,1-3H3,(H2,54,70)(H,56,71)(H,57,64)(H,58,65)(H,59,73)(H,60,74)(H,61,76)(H,62,75)(H,63,72)(H,66,67)(H,68,69)(H,77,78,79)/t37-,38-,39-,40-,41-,42-,43-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a |
Hadassah-University Hospital
Curated by ChEMBL
| Assay Description
In vitro smooth muscle contraction activity in guinea pig ileum consists of two or three CCK receptor subtypes |
J Med Chem 35: 2806-11 (1992)
BindingDB Entry DOI: 10.7270/Q28C9V6B |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Roche Products Ltd
Curated by ChEMBL
| Assay Description
Concentration of compound that induced 50% of the maximal contraction in guinea pig gallbladder tissue when tested in vitro |
Bioorg Med Chem Lett 7: 429-32 (1997)
Article DOI: 10.1016/S0960-894X(97)00062-0
BindingDB Entry DOI: 10.7270/Q2DJ5G4B |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50004481

(CCK7 analogue | CHEMBL99499)Show SMILES CCCC[C@H](NC(=O)CCc1ccc(OS(O)(=O)=O)cc1)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCC)C(=O)N[C@@H](CC(O)=O)C(=O)N\C(=C/c1ccccc1)C(N)=O Show InChI InChI=1S/C47H58N8O13S/c1-3-5-15-35(51-40(56)23-20-29-18-21-32(22-19-29)68-69(65,66)67)44(61)50-28-41(57)52-38(25-31-27-49-34-17-11-10-14-33(31)34)46(63)53-36(16-6-4-2)45(62)55-39(26-42(58)59)47(64)54-37(43(48)60)24-30-12-8-7-9-13-30/h7-14,17-19,21-22,24,27,35-36,38-39,49H,3-6,15-16,20,23,25-26,28H2,1-2H3,(H2,48,60)(H,50,61)(H,51,56)(H,52,57)(H,53,63)(H,54,64)(H,55,62)(H,58,59)(H,65,66,67)/b37-24-/t35-,36-,38-,39-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Dissociation rate constant of compound for mutant T46S Escherichia coli dihydrofolate reductase |
J Med Chem 35: 2919-28 (1992)
BindingDB Entry DOI: 10.7270/Q2W66JRH |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50004439
(3-Acetylamino-N-[1-[1-({[1-{1-[1-(1-carbamoyl-2-ph...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)N(C)C(=O)[C@H](CC(O)=O)NC(C)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C54H70N10O17S/c1-5-7-17-38(61-53(76)44(25-33-20-22-35(23-21-33)81-82(78,79)80)64(4)54(77)43(28-47(69)70)58-31(3)65)49(72)57-30-45(66)59-41(26-34-29-56-37-19-13-12-16-36(34)37)51(74)60-39(18-8-6-2)50(73)63-42(27-46(67)68)52(75)62-40(48(55)71)24-32-14-10-9-11-15-32/h9-16,19-23,29,38-44,56H,5-8,17-18,24-28,30H2,1-4H3,(H2,55,71)(H,57,72)(H,58,65)(H,59,66)(H,60,74)(H,61,76)(H,62,75)(H,63,73)(H,67,68)(H,69,70)(H,78,79,80)/t38-,39-,40-,41-,42-,43-,44-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a |
Hadassah-University Hospital
Curated by ChEMBL
| Assay Description
In vitro smooth muscle contraction activity in guinea pig ileum consists of two or three CCK receptor subtypes |
J Med Chem 35: 2806-11 (1992)
BindingDB Entry DOI: 10.7270/Q28C9V6B |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50004475
(CCK7 analogue | CHEMBL439591 | N-(1-Carbamoyl-2-ph...)Show SMILES CCCC[C@H](NC(=O)CCc1ccc(OS(O)(=O)=O)cc1)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C47H60N8O13S/c1-4-5-14-35(51-40(56)21-18-29-16-19-32(20-17-29)68-69(65,66)67)44(61)50-27-41(57)52-38(24-31-26-49-34-15-10-9-13-33(31)34)46(63)54-37(22-28(2)3)45(62)55-39(25-42(58)59)47(64)53-36(43(48)60)23-30-11-7-6-8-12-30/h6-13,15-17,19-20,26,28,35-39,49H,4-5,14,18,21-25,27H2,1-3H3,(H2,48,60)(H,50,61)(H,51,56)(H,52,57)(H,53,64)(H,54,63)(H,55,62)(H,58,59)(H,65,66,67)/t35-,36-,37-,38-,39-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Thermodynamic dissociation constant of compound for mutant T46N E. coli dihydrofolate reductase |
J Med Chem 35: 2919-28 (1992)
BindingDB Entry DOI: 10.7270/Q2W66JRH |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50004489
(CCK7 analogue | CHEMBL318010)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CNC(=O)CNC(=O)CCc1ccc(OS(O)(=O)=O)cc1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C43H52N8O13S/c1-2-3-12-32(41(58)51-35(22-39(55)56)43(60)50-33(40(44)57)20-27-9-5-4-6-10-27)49-42(59)34(21-28-23-45-31-13-8-7-11-30(28)31)48-38(54)25-47-37(53)24-46-36(52)19-16-26-14-17-29(18-15-26)64-65(61,62)63/h4-11,13-15,17-18,23,32-35,45H,2-3,12,16,19-22,24-25H2,1H3,(H2,44,57)(H,46,52)(H,47,53)(H,48,54)(H,49,59)(H,50,60)(H,51,58)(H,55,56)(H,61,62,63)/t32-,33-,34-,35-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Thermodynamic dissociation constant of compound for wild type E. coli dihydrofolate reductase |
J Med Chem 35: 2919-28 (1992)
BindingDB Entry DOI: 10.7270/Q2W66JRH |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50004438
(3-Acetylamino-N-[1-[1-({[1-(1-{1-[(1-carbamoyl-2-p...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@H](CC(O)=O)NC(C)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCC)C(=O)N[C@@H](CC(O)=O)C(=O)N(C)[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C54H70N10O17S/c1-5-7-17-38(60-51(74)40(62-53(76)42(27-46(67)68)58-31(3)65)24-33-20-22-35(23-21-33)81-82(78,79)80)49(72)57-30-45(66)59-41(26-34-29-56-37-19-13-12-16-36(34)37)52(75)61-39(18-8-6-2)50(73)63-43(28-47(69)70)54(77)64(4)44(48(55)71)25-32-14-10-9-11-15-32/h9-16,19-23,29,38-44,56H,5-8,17-18,24-28,30H2,1-4H3,(H2,55,71)(H,57,72)(H,58,65)(H,59,66)(H,60,74)(H,61,75)(H,62,76)(H,63,73)(H,67,68)(H,69,70)(H,78,79,80)/t38-,39-,40-,41-,42-,43-,44-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
Hadassah-University Hospital
Curated by ChEMBL
| Assay Description
In vitro smooth muscle contraction activity in guinea pig ileum consists of two or three CCK receptor subtypes |
J Med Chem 35: 2806-11 (1992)
BindingDB Entry DOI: 10.7270/Q28C9V6B |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50455609
(CHEMBL2310857)Show SMILES CCCC[C@H](NC(=O)CCc1ccc(OS(O)(=O)=O)cc1)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCC)C(=O)N1CC(C[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(N)=O)OS(O)(=O)=O |r| Show InChI InChI=1S/C48H62N8O15S2/c1-3-5-15-37(52-42(57)23-20-30-18-21-33(22-19-30)70-72(64,65)66)45(60)51-28-43(58)53-40(25-32-27-50-36-17-11-10-14-35(32)36)46(61)54-38(16-6-4-2)48(63)56-29-34(71-73(67,68)69)26-41(56)47(62)55-39(44(49)59)24-31-12-8-7-9-13-31/h7-14,17-19,21-22,27,34,37-41,50H,3-6,15-16,20,23-26,28-29H2,1-2H3,(H2,49,59)(H,51,60)(H,52,57)(H,53,58)(H,54,61)(H,55,62)(H,64,65,66)(H,67,68,69)/t34?,37-,38-,39-,40-,41-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Dissociation rate constant of compound for mutant T46N Escherichia coli dihydrofolate reductase |
J Med Chem 35: 2919-28 (1992)
BindingDB Entry DOI: 10.7270/Q2W66JRH |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50004490

(CCK7 analogue | CHEMBL412238 | N-(1-Carbamoyl-2-ph...)Show SMILES CCCC[C@H](NC(=O)CCc1ccc(OS(O)(=O)=O)cc1)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N(C)[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C48H62N8O13S/c1-5-6-15-36(52-41(57)22-19-30-17-20-33(21-18-30)69-70(66,67)68)45(62)51-28-42(58)53-39(25-32-27-50-35-16-11-10-14-34(32)35)48(65)56(4)40(23-29(2)3)47(64)55-38(26-43(59)60)46(63)54-37(44(49)61)24-31-12-8-7-9-13-31/h7-14,16-18,20-21,27,29,36-40,50H,5-6,15,19,22-26,28H2,1-4H3,(H2,49,61)(H,51,62)(H,52,57)(H,53,58)(H,54,63)(H,55,64)(H,59,60)(H,66,67,68)/t36-,37-,38-,39-,40-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitor constant of compound for mutant T46N E. coli dihydrofolate reductase |
J Med Chem 35: 2919-28 (1992)
BindingDB Entry DOI: 10.7270/Q2W66JRH |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50004435

(3-Acetylamino-N-[1-{1-[({[1-{1-[1-(1-carbamoyl-2-p...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@H](CC(O)=O)NC(C)=O)C(=O)NCC(=O)N(C)[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C54H70N10O17S/c1-5-7-17-38(59-51(74)41(62-52(75)42(27-46(67)68)58-31(3)65)25-33-20-22-35(23-21-33)81-82(78,79)80)49(72)57-30-45(66)64(4)44(26-34-29-56-37-19-13-12-16-36(34)37)54(77)60-39(18-8-6-2)50(73)63-43(28-47(69)70)53(76)61-40(48(55)71)24-32-14-10-9-11-15-32/h9-16,19-23,29,38-44,56H,5-8,17-18,24-28,30H2,1-4H3,(H2,55,71)(H,57,72)(H,58,65)(H,59,74)(H,60,77)(H,61,76)(H,62,75)(H,63,73)(H,67,68)(H,69,70)(H,78,79,80)/t38-,39-,40-,41-,42-,43-,44-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 26 | n/a | n/a | n/a | n/a |
Hadassah-University Hospital
Curated by ChEMBL
| Assay Description
In vitro smooth muscle contraction activity in guinea pig ileum consists of two or three CCK receptor subtypes |
J Med Chem 35: 2806-11 (1992)
BindingDB Entry DOI: 10.7270/Q28C9V6B |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50472279
(CHEMBL405243)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccc(cc1)C(=O)c1ccccc1)NC(=O)[C@H](CCCC)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(O)=O Show InChI InChI=1S/C76H87N11O18/c1-3-5-20-56(81-71(99)58(37-47-27-33-52(89)34-28-47)84-74(102)61(40-65(91)92)80-64(90)43-79-68(96)54(77)35-45-25-31-51(88)32-26-45)69(97)83-59(36-46-23-29-49(30-24-46)67(95)48-17-11-8-12-18-48)72(100)85-60(39-50-42-78-55-22-14-13-19-53(50)55)73(101)82-57(21-6-4-2)70(98)86-62(41-66(93)94)75(103)87-63(76(104)105)38-44-15-9-7-10-16-44/h7-19,22-34,42,54,56-63,78,88-89H,3-6,20-21,35-41,43,77H2,1-2H3,(H,79,96)(H,80,90)(H,81,99)(H,82,101)(H,83,97)(H,84,102)(H,85,100)(H,86,98)(H,87,103)(H,91,92)(H,93,94)(H,104,105)/t54-,56+,57+,58+,59+,60+,61+,62+,63+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 28 | n/a | n/a | n/a | n/a |
Mayo Clinic and Foundation
Curated by ChEMBL
| Assay Description
Ability to stimulate intracellular calcium response was determined using CCK receptor-bearing chinese hamster ovary cell line |
J Med Chem 42: 2105-11 (1999)
Article DOI: 10.1021/jm980732q
BindingDB Entry DOI: 10.7270/Q27P924Q |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50004433
(3-Acetylamino-N-[1-[1-({[1-({1-[1-(1-carbamoyl-2-p...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@H](CC(O)=O)NC(C)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N(C)[C@@H](CCCC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C54H70N10O17S/c1-5-7-17-38(60-50(73)40(62-51(74)41(27-46(67)68)58-31(3)65)25-33-20-22-35(23-21-33)81-82(78,79)80)49(72)57-30-45(66)59-43(26-34-29-56-37-18-13-12-16-36(34)37)54(77)64(4)44(19-8-6-2)53(76)63-42(28-47(69)70)52(75)61-39(48(55)71)24-32-14-10-9-11-15-32/h9-16,18,20-23,29,38-44,56H,5-8,17,19,24-28,30H2,1-4H3,(H2,55,71)(H,57,72)(H,58,65)(H,59,66)(H,60,73)(H,61,75)(H,62,74)(H,63,76)(H,67,68)(H,69,70)(H,78,79,80)/t38-,39-,40-,41-,42-,43-,44-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 37 | n/a | n/a | n/a | n/a |
Hadassah-University Hospital
Curated by ChEMBL
| Assay Description
In vitro smooth muscle contraction activity in guinea pig ileum consists of two or three CCK receptor subtypes |
J Med Chem 35: 2806-11 (1992)
BindingDB Entry DOI: 10.7270/Q28C9V6B |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50004488
(CCK7 analogue | CHEMBL414793)Show SMILES CCCC[C@H](NC(=O)CCc1ccc(OS(O)(=O)=O)cc1)C(=O)N[C@H](C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C48H62N8O13S/c1-4-6-16-36(52-41(57)24-21-30-19-22-33(23-20-30)69-70(66,67)68)45(62)51-29(3)44(61)55-39(26-32-28-50-35-18-12-11-15-34(32)35)47(64)53-37(17-7-5-2)46(63)56-40(27-42(58)59)48(65)54-38(43(49)60)25-31-13-9-8-10-14-31/h8-15,18-20,22-23,28-29,36-40,50H,4-7,16-17,21,24-27H2,1-3H3,(H2,49,60)(H,51,62)(H,52,57)(H,53,64)(H,54,65)(H,55,61)(H,56,63)(H,58,59)(H,66,67,68)/t29-,36+,37+,38+,39+,40+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 50 | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Dissociation rate constant of compound for mutant T46S Escherichia coli dihydrofolate reductase |
J Med Chem 35: 2919-28 (1992)
BindingDB Entry DOI: 10.7270/Q2W66JRH |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50004492

(CCK7 analogue | CHEMBL98720)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CN1CC[C@H](NC(=O)CCc2ccc(OS(O)(=O)=O)cc2)C1=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C45H54N8O13S/c1-2-3-12-33(42(59)52-37(24-40(56)57)44(61)51-35(41(46)58)22-28-9-5-4-6-10-28)50-43(60)36(23-29-25-47-32-13-8-7-11-31(29)32)49-39(55)26-53-21-20-34(45(53)62)48-38(54)19-16-27-14-17-30(18-15-27)66-67(63,64)65/h4-11,13-15,17-18,25,33-37,47H,2-3,12,16,19-24,26H2,1H3,(H2,46,58)(H,48,54)(H,49,55)(H,50,60)(H,51,61)(H,52,59)(H,56,57)(H,63,64,65)/t33-,34-,35-,36-,37-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 71 | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Dissociation rate constant of compound for wild type Escherichia coli dihydrofolate reductase |
J Med Chem 35: 2919-28 (1992)
BindingDB Entry DOI: 10.7270/Q2W66JRH |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50004434

(3-Acetylamino-N-[1-(1-{2-[1-{1-[1-(1-carbamoyl-2-p...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCC)NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@H](CC(O)=O)NC(C)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C56H72N10O17S/c1-4-6-17-39(50(73)64-45(30-48(70)71)54(77)62-41(49(57)72)26-33-14-9-8-10-15-33)60-52(75)43(28-35-31-58-38-19-12-11-16-37(35)38)65-55(78)46-20-13-25-66(46)56(79)40(18-7-5-2)61-51(74)42(63-53(76)44(29-47(68)69)59-32(3)67)27-34-21-23-36(24-22-34)83-84(80,81)82/h8-12,14-16,19,21-24,31,39-46,58H,4-7,13,17-18,20,25-30H2,1-3H3,(H2,57,72)(H,59,67)(H,60,75)(H,61,74)(H,62,77)(H,63,76)(H,64,73)(H,65,78)(H,68,69)(H,70,71)(H,80,81,82)/t39-,40-,41-,42-,43-,44-,45-,46-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 104 | n/a | n/a | n/a | n/a |
Hadassah-University Hospital
Curated by ChEMBL
| Assay Description
In vitro smooth muscle contraction activity in guinea pig ileum consists of two or three CCK receptor subtypes |
J Med Chem 35: 2806-11 (1992)
BindingDB Entry DOI: 10.7270/Q28C9V6B |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50450536
(CHEMBL357983)Show SMILES [H][C@](CCC(O)=O)(NC(=O)Nc1ccccc1)C(=O)N(CC(=O)N(C(C)C)c1ccc(OC)cc1)c1ccccc1 Show InChI InChI=1S/C30H34N4O6/c1-21(2)34(24-14-16-25(40-3)17-15-24)27(35)20-33(23-12-8-5-9-13-23)29(38)26(18-19-28(36)37)32-30(39)31-22-10-6-4-7-11-22/h4-17,21,26H,18-20H2,1-3H3,(H,36,37)(H2,31,32,39)/t26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a |
Roche Products Ltd
Curated by ChEMBL
| Assay Description
Concentration of compound that induced 50% of the maximal contraction in guinea pig gallbladder tissue when tested in vitro |
Bioorg Med Chem Lett 7: 429-32 (1997)
Article DOI: 10.1016/S0960-894X(97)00062-0
BindingDB Entry DOI: 10.7270/Q2DJ5G4B |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50004485

(CCK7 analogue | CHEMBL98631)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CCCCNC(=O)CCc1ccc(OS(O)(=O)=O)cc1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C44H55N7O12S/c1-27(2)22-35(42(57)51-37(25-40(54)55)44(59)49-34(41(45)56)23-29-10-4-3-5-11-29)50-43(58)36(24-30-26-47-33-13-7-6-12-32(30)33)48-39(53)14-8-9-21-46-38(52)20-17-28-15-18-31(19-16-28)63-64(60,61)62/h3-7,10-13,15-16,18-19,26-27,34-37,47H,8-9,14,17,20-25H2,1-2H3,(H2,45,56)(H,46,52)(H,48,53)(H,49,59)(H,50,58)(H,51,57)(H,54,55)(H,60,61,62)/t34-,35-,36-,37-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 140 | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Dissociation rate constant of compound for mutant T46N Escherichia coli dihydrofolate reductase |
J Med Chem 35: 2919-28 (1992)
BindingDB Entry DOI: 10.7270/Q2W66JRH |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50472278
(CHEMBL410965)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccc(cc1)C(=O)c1ccccc1)NC(=O)[C@H](CCCC)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@H](N)Cc1ccc(O)cc1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccc(cc1)C(=O)c1ccccc1)C(O)=O Show InChI InChI=1S/C83H91N11O19/c1-3-5-20-62(88-78(107)64(41-51-29-37-58(96)38-30-51)91-81(110)67(44-71(98)99)87-70(97)47-86-75(104)60(84)39-48-27-35-57(95)36-28-48)76(105)90-65(40-49-23-31-54(32-24-49)73(102)52-15-9-7-10-16-52)79(108)92-66(43-56-46-85-61-22-14-13-19-59(56)61)80(109)89-63(21-6-4-2)77(106)93-68(45-72(100)101)82(111)94-69(83(112)113)42-50-25-33-55(34-26-50)74(103)53-17-11-8-12-18-53/h7-19,22-38,46,60,62-69,85,95-96H,3-6,20-21,39-45,47,84H2,1-2H3,(H,86,104)(H,87,97)(H,88,107)(H,89,109)(H,90,105)(H,91,110)(H,92,108)(H,93,106)(H,94,111)(H,98,99)(H,100,101)(H,112,113)/t60-,62+,63+,64+,65+,66+,67+,68+,69+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 150 | n/a | n/a | n/a | n/a |
Mayo Clinic and Foundation
Curated by ChEMBL
| Assay Description
Ability to stimulate intracellular calcium response was determined using CCK receptor-bearing chinese hamster ovary cell line |
J Med Chem 42: 2105-11 (1999)
Article DOI: 10.1021/jm980732q
BindingDB Entry DOI: 10.7270/Q27P924Q |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50450537
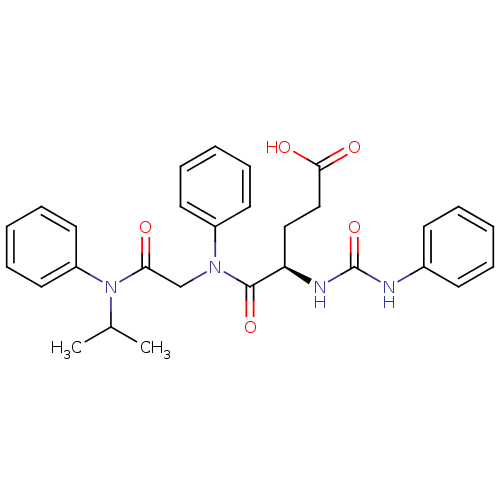
(CHEMBL146206)Show SMILES [H][C@](CCC(O)=O)(NC(=O)Nc1ccccc1)C(=O)N(CC(=O)N(C(C)C)c1ccccc1)c1ccccc1 Show InChI InChI=1S/C29H32N4O5/c1-21(2)33(24-16-10-5-11-17-24)26(34)20-32(23-14-8-4-9-15-23)28(37)25(18-19-27(35)36)31-29(38)30-22-12-6-3-7-13-22/h3-17,21,25H,18-20H2,1-2H3,(H,35,36)(H2,30,31,38)/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | n/a | 210 | n/a | n/a | n/a | n/a |
Roche Products Ltd
Curated by ChEMBL
| Assay Description
Concentration of compound that induced 50% of the maximal contraction in guinea pig gallbladder tissue when tested in vitro |
Bioorg Med Chem Lett 7: 429-32 (1997)
Article DOI: 10.1016/S0960-894X(97)00062-0
BindingDB Entry DOI: 10.7270/Q2DJ5G4B |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50472277

(CHEMBL438912)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccc(cc1)C(=O)c1ccccc1)C(O)=O Show InChI InChI=1S/C69H81N11O18/c1-3-5-15-50(76-65(93)52(31-41-22-28-46(82)29-23-41)78-67(95)54(34-59(85)86)75-57(83)37-72-62(90)48(70)30-39-20-26-45(81)27-21-39)63(91)73-38-58(84)74-53(33-44-36-71-49-17-11-10-14-47(44)49)66(94)77-51(16-6-4-2)64(92)79-55(35-60(87)88)68(96)80-56(69(97)98)32-40-18-24-43(25-19-40)61(89)42-12-8-7-9-13-42/h7-14,17-29,36,48,50-56,71,81-82H,3-6,15-16,30-35,37-38,70H2,1-2H3,(H,72,90)(H,73,91)(H,74,84)(H,75,83)(H,76,93)(H,77,94)(H,78,95)(H,79,92)(H,80,96)(H,85,86)(H,87,88)(H,97,98)/t48-,50+,51+,52+,53+,54+,55+,56+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 280 | n/a | n/a | n/a | n/a |
Mayo Clinic and Foundation
Curated by ChEMBL
| Assay Description
Ability to stimulate intracellular calcium response was determined using CCK receptor-bearing chinese hamster ovary cell line |
J Med Chem 42: 2105-11 (1999)
Article DOI: 10.1021/jm980732q
BindingDB Entry DOI: 10.7270/Q27P924Q |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50450535
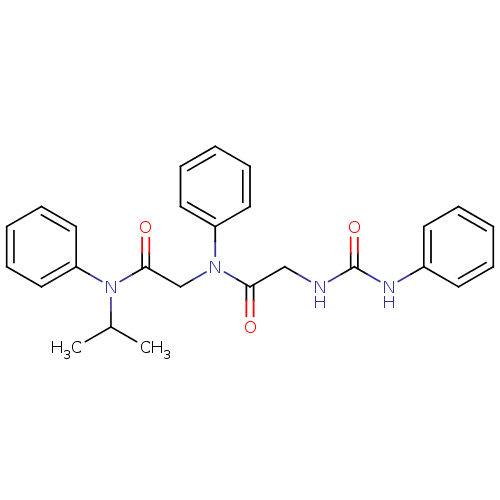
(CHEMBL421778)Show SMILES CC(C)N(C(=O)CN(C(=O)CNC(=O)Nc1ccccc1)c1ccccc1)c1ccccc1 Show InChI InChI=1S/C26H28N4O3/c1-20(2)30(23-16-10-5-11-17-23)25(32)19-29(22-14-8-4-9-15-22)24(31)18-27-26(33)28-21-12-6-3-7-13-21/h3-17,20H,18-19H2,1-2H3,(H2,27,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a |
Roche Products Ltd
Curated by ChEMBL
| Assay Description
Concentration of compound that induced 50% of the maximal contraction in guinea pig gallbladder tissue when tested in vitro |
Bioorg Med Chem Lett 7: 429-32 (1997)
Article DOI: 10.1016/S0960-894X(97)00062-0
BindingDB Entry DOI: 10.7270/Q2DJ5G4B |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50072427

(1-(1-(2-(isopropyl(phenyl)amino)-2-oxoethyl)-2,4-d...)Show SMILES CC(C)N(C(=O)CN1c2ccccc2N(c2ccccc2)C(=O)C(NC(=O)Nc2ccccc2)C1=O)c1ccccc1 Show InChI InChI=1S/C33H31N5O4/c1-23(2)37(25-16-8-4-9-17-25)29(39)22-36-27-20-12-13-21-28(27)38(26-18-10-5-11-19-26)32(41)30(31(36)40)35-33(42)34-24-14-6-3-7-15-24/h3-21,23,30H,22H2,1-2H3,(H2,34,35,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a |
Roche Products Ltd
Curated by ChEMBL
| Assay Description
Concentration of compound that induced 50% of the maximal contraction in guinea pig gallbladder tissue when tested in vitro |
Bioorg Med Chem Lett 7: 429-32 (1997)
Article DOI: 10.1016/S0960-894X(97)00062-0
BindingDB Entry DOI: 10.7270/Q2DJ5G4B |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50215056
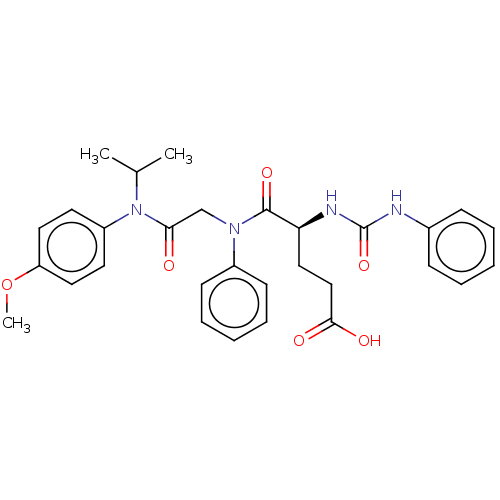
(CHEMBL346160)Show SMILES [H][C@@](CCC(O)=O)(NC(=O)Nc1ccccc1)C(=O)N(CC(=O)N(C(C)C)c1ccc(OC)cc1)c1ccccc1 Show InChI InChI=1S/C30H34N4O6/c1-21(2)34(24-14-16-25(40-3)17-15-24)27(35)20-33(23-12-8-5-9-13-23)29(38)26(18-19-28(36)37)32-30(39)31-22-10-6-4-7-11-22/h4-17,21,26H,18-20H2,1-3H3,(H,36,37)(H2,31,32,39)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a |
Roche Products Ltd
Curated by ChEMBL
| Assay Description
Concentration of compound that induced 50% of the maximal contraction in guinea pig gallbladder tissue when tested in vitro |
Bioorg Med Chem Lett 7: 429-32 (1997)
Article DOI: 10.1016/S0960-894X(97)00062-0
BindingDB Entry DOI: 10.7270/Q2DJ5G4B |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data