 Found 1046 hits of ic50 for UniProtKB: P10980
Found 1046 hits of ic50 for UniProtKB: P10980 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50213281
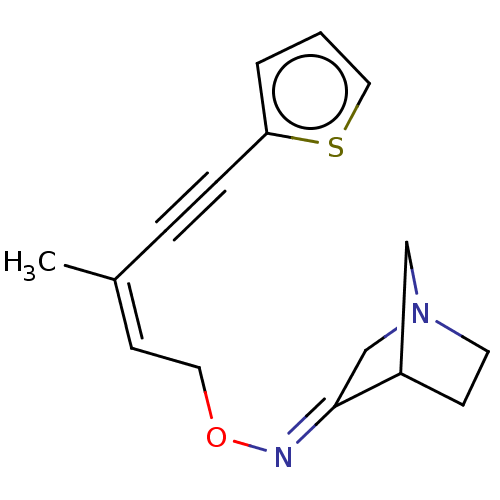
(CHEMBL351561)Show SMILES C\C(=C\CO\N=C1/CN2CCC1C2)C#Cc1cccs1 |THB:5:6:12:10.9| Show InChI InChI=1S/C16H18N2OS/c1-13(4-5-15-3-2-10-20-15)7-9-19-17-16-12-18-8-6-14(16)11-18/h2-3,7,10,14H,6,8-9,11-12H2,1H3/b13-7-,17-16+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity against muscarinic receptor using radioligand [3H]cis-methyldioxolane (CMD) binding assay in membrane preparations of rat neocortex. |
Citation and Details
BindingDB Entry DOI: 10.7270/Q23B628P |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50213074
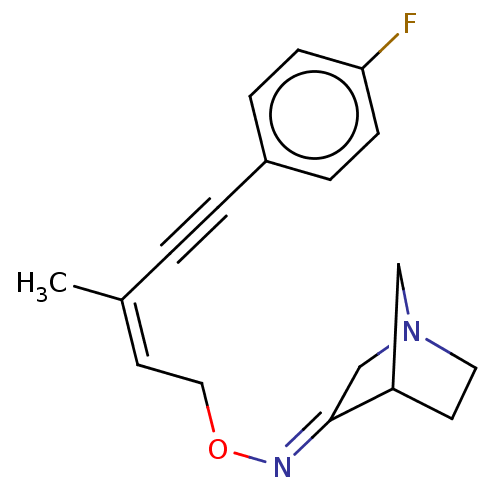
(CHEMBL156050)Show SMILES C\C(=C\CO\N=C1/CN2CCC1C2)C#Cc1ccc(F)cc1 |THB:5:6:12:10.9| Show InChI InChI=1S/C18H19FN2O/c1-14(2-3-15-4-6-17(19)7-5-15)9-11-22-20-18-13-21-10-8-16(18)12-21/h4-7,9,16H,8,10-13H2,1H3/b14-9-,20-18+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity against muscarinic receptor using radioligand [3H]cis-methyldioxolane (CMD) binding assay in membrane preparations of rat neocortex. |
Citation and Details
BindingDB Entry DOI: 10.7270/Q23B628P |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50213283
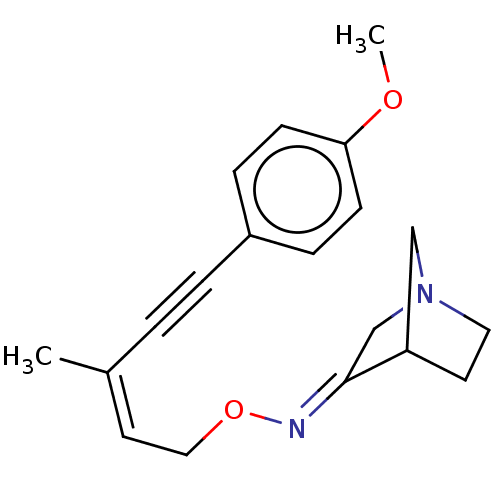
(CHEMBL350223)Show SMILES COc1ccc(cc1)C#C\C(C)=C/CO\N=C1/CN2CCC1C2 |THB:15:16:22:20.19| Show InChI InChI=1S/C19H22N2O2/c1-15(3-4-16-5-7-18(22-2)8-6-16)10-12-23-20-19-14-21-11-9-17(19)13-21/h5-8,10,17H,9,11-14H2,1-2H3/b15-10-,20-19+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity against muscarinic receptor using radioligand [3H]cis-methyldioxolane (CMD) binding assay in membrane preparations of rat neocortex. |
Citation and Details
BindingDB Entry DOI: 10.7270/Q23B628P |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50213207
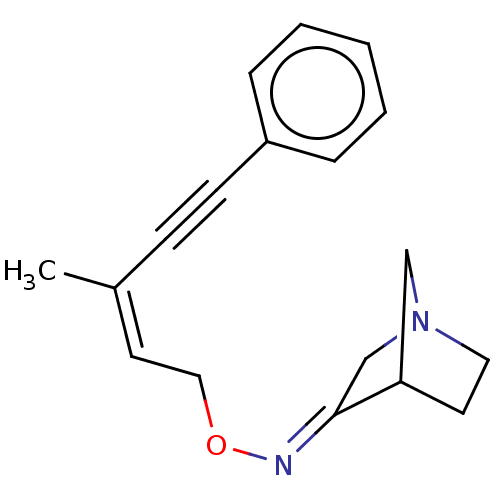
(CHEMBL160582)Show SMILES C\C(=C\CO\N=C1/CN2CCC1C2)C#Cc1ccccc1 |THB:5:6:12:10.9| Show InChI InChI=1S/C18H20N2O/c1-15(7-8-16-5-3-2-4-6-16)10-12-21-19-18-14-20-11-9-17(18)13-20/h2-6,10,17H,9,11-14H2,1H3/b15-10-,19-18+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity against muscarinic receptor using radioligand [3H]cis-methyldioxolane (CMD) binding assay in membrane preparations of rat neocortex. |
Citation and Details
BindingDB Entry DOI: 10.7270/Q23B628P |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50280565
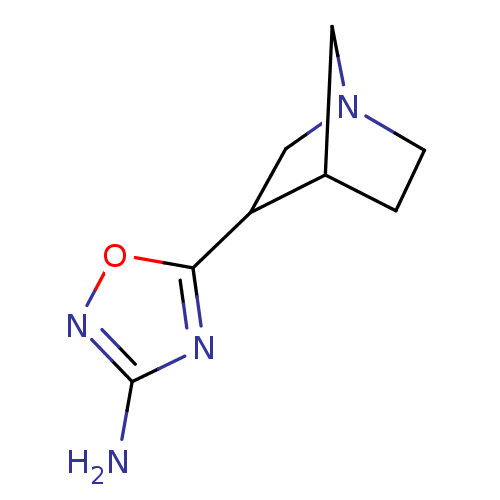
(5-(1-Aza-bicyclo[2.2.1]hept-3-yl)-[1,2,4]oxadiazol...)Show InChI InChI=1S/C8H12N4O/c9-8-10-7(13-11-8)6-4-12-2-1-5(6)3-12/h5-6H,1-4H2,(H2,9,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of [3H]cis-methyldioxolane binding to label agonist sites (RCMD) in rat neocortex |
Bioorg Med Chem Lett 2: 821-826 (1992)
Article DOI: 10.1016/S0960-894X(00)80538-7
BindingDB Entry DOI: 10.7270/Q2PG1RNN |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50213070
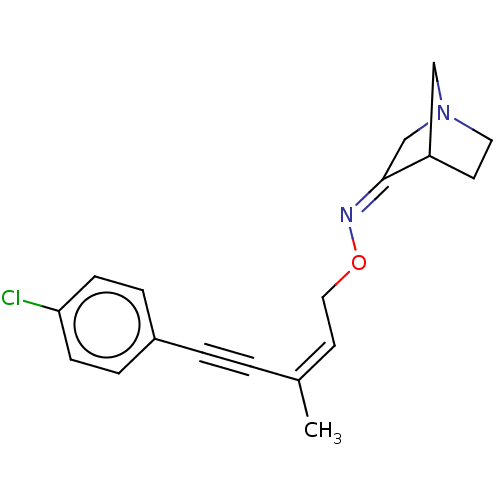
(CHEMBL405426)Show SMILES C\C(=C\CO\N=C1\CN2CCC1C2)C#Cc1ccc(Cl)cc1 |THB:5:6:12:10.9| Show InChI InChI=1S/C18H19ClN2O/c1-14(2-3-15-4-6-17(19)7-5-15)9-11-22-20-18-13-21-10-8-16(18)12-21/h4-7,9,16H,8,10-13H2,1H3/b14-9-,20-18- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity against muscarinic receptor using radioligand [3H]cis-methyldioxolane (CMD) binding assay in membrane preparations of rat neocortex. |
Citation and Details
BindingDB Entry DOI: 10.7270/Q23B628P |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50213278
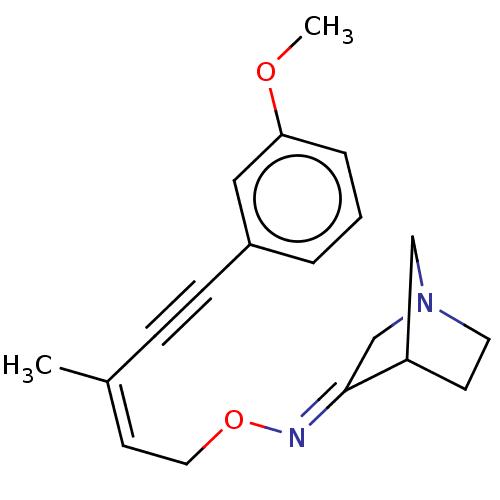
(CHEMBL156445)Show SMILES COc1cccc(c1)C#C\C(C)=C/CO\N=C1/CN2CCC1C2 |THB:15:16:22:20.19| Show InChI InChI=1S/C19H22N2O2/c1-15(6-7-16-4-3-5-18(12-16)22-2)9-11-23-20-19-14-21-10-8-17(19)13-21/h3-5,9,12,17H,8,10-11,13-14H2,1-2H3/b15-9-,20-19+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity against muscarinic receptor using radioligand [3H]cis-methyldioxolane (CMD) binding assay in membrane preparations of rat neocortex. |
Citation and Details
BindingDB Entry DOI: 10.7270/Q23B628P |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50213077
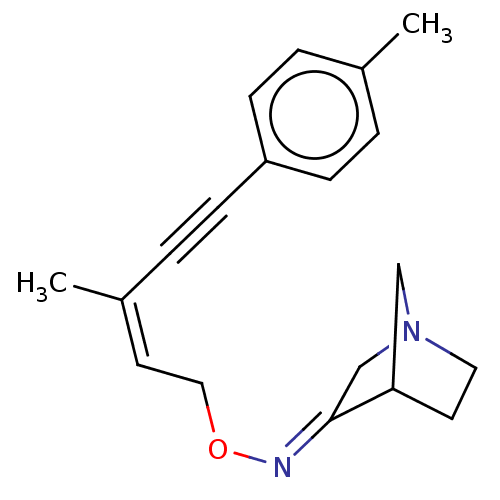
(CHEMBL348412)Show SMILES C\C(=C\CO\N=C1/CN2CCC1C2)C#Cc1ccc(C)cc1 |THB:5:6:12:10.9| Show InChI InChI=1S/C19H22N2O/c1-15-3-6-17(7-4-15)8-5-16(2)10-12-22-20-19-14-21-11-9-18(19)13-21/h3-4,6-7,10,18H,9,11-14H2,1-2H3/b16-10-,20-19+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity against muscarinic receptor using radioligand [3H]cis-methyldioxolane (CMD) binding assay in membrane preparations of rat neocortex. |
Citation and Details
BindingDB Entry DOI: 10.7270/Q23B628P |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50070692
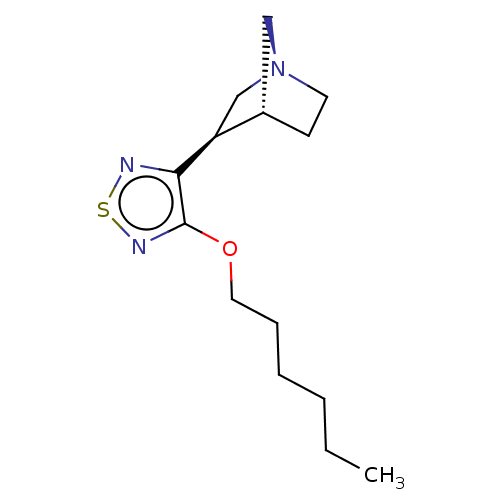
(CHEMBL99240)Show SMILES [H][C@@]12CC[N@@](C[C@@H]1c1nsnc1OCCCCCC)C2 Show InChI InChI=1S/C34H50N8O8/c1-3-20(2)28(42-30(46)24(12-7-8-16-35)39-29(45)23-19-22(43)14-15-27(23)44)32(48)41-26(18-21-10-5-4-6-11-21)31(47)40-25(33(49)50)13-9-17-38-34(36)37/h4-6,10-11,14-15,19-20,24-26,28,43-44H,3,7-9,12-13,16-18,35H2,1-2H3,(H,39,45)(H,40,47)(H,41,48)(H,42,46)(H,49,50)(H4,36,37,38)/t20?,24-,25-,26-,28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50072214
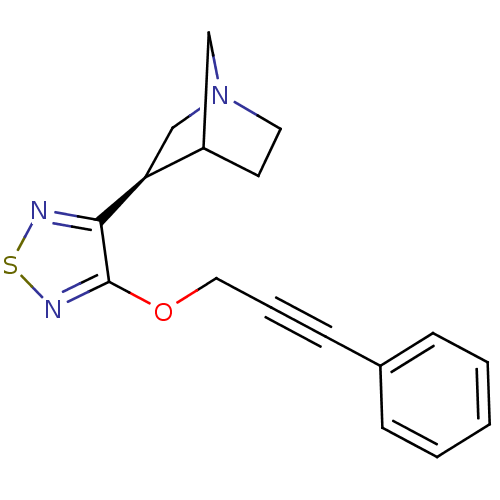
((R)-3-[4-(3-Phenyl-prop-2-ynyloxy)-[1,2,5]thiadiaz...)Show InChI InChI=1S/C17H17N3OS/c1-2-5-13(6-3-1)7-4-10-21-17-16(18-22-19-17)15-12-20-9-8-14(15)11-20/h1-3,5-6,14-15H,8-12H2/t14?,15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50291551

(CHEMBL274329 | Trimethyl-[4-(3-oxo-isoxazolidin-2-...)Show InChI InChI=1S/C10H17N2O2/c1-12(2,3)8-5-4-7-11-10(13)6-9-14-11/h6-9H2,1-3H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Muscarinic receptor M2 in rat heart using [3H]-QNB (quinuclidinyl benzylate) radioligand as a M2 non-selective muscarinic receptor antagonist at a co... |
Bioorg Med Chem Lett 7: 1033-1036 (1997)
Article DOI: 10.1016/S0960-894X(97)00150-9
BindingDB Entry DOI: 10.7270/Q2S46RZ2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50213286

(CHEMBL346580)Show SMILES C\C(=C/CO\N=C1/CN2CCC1C2)C#Cc1ccc(Cl)c(Cl)c1 |THB:5:6:12:10.9| Show InChI InChI=1S/C18H18Cl2N2O/c1-13(2-3-14-4-5-16(19)17(20)10-14)7-9-23-21-18-12-22-8-6-15(18)11-22/h4-5,7,10,15H,6,8-9,11-12H2,1H3/b13-7+,21-18+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity against muscarinic receptor using radioligand [3H]cis-methyldioxolane (CMD) binding assay in membrane preparations of rat neocortex. |
Citation and Details
BindingDB Entry DOI: 10.7270/Q23B628P |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50065207
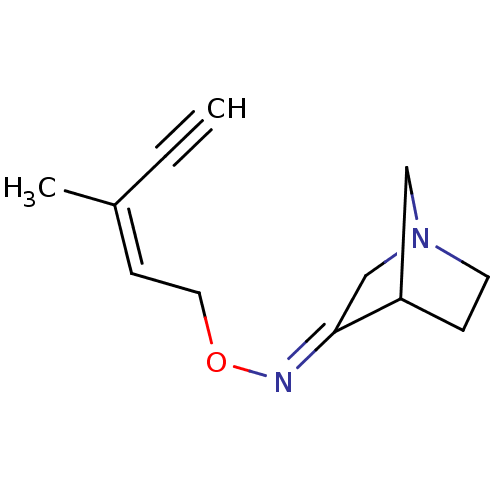
(1-Aza-bicyclo[2.2.1]heptan-3-one O-((Z)-3-methyl-p...)Show InChI InChI=1S/C12H16N2O/c1-3-10(2)5-7-15-13-12-9-14-6-4-11(12)8-14/h1,5,11H,4,6-9H2,2H3/b10-5-,13-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity against muscarinic receptor using radioligand [3H]cis-methyldioxolane (CMD) binding assay in membrane preparations of rat neocortex. |
Citation and Details
BindingDB Entry DOI: 10.7270/Q23B628P |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
In vitro displacement of [3H]oxotremorine-M from muscarinic acetylcholine receptor in rat brain cortex |
J Med Chem 42: 4970-80 (1999)
Article DOI: 10.1021/jm9910627
BindingDB Entry DOI: 10.7270/Q2K0770F |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50213279

(CHEMBL345415)Show SMILES C\C(=C\CO\N=C1\CN2CCC1C2)C#Cc1ccccc1 |THB:5:6:12:10.9| Show InChI InChI=1S/C18H20N2O/c1-15(7-8-16-5-3-2-4-6-16)10-12-21-19-18-14-20-11-9-17(18)13-20/h2-6,10,17H,9,11-14H2,1H3/b15-10-,19-18- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity against muscarinic receptor using radioligand [3H]cis-methyldioxolane (CMD) binding assay in membrane preparations of rat neocortex. |
Citation and Details
BindingDB Entry DOI: 10.7270/Q23B628P |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
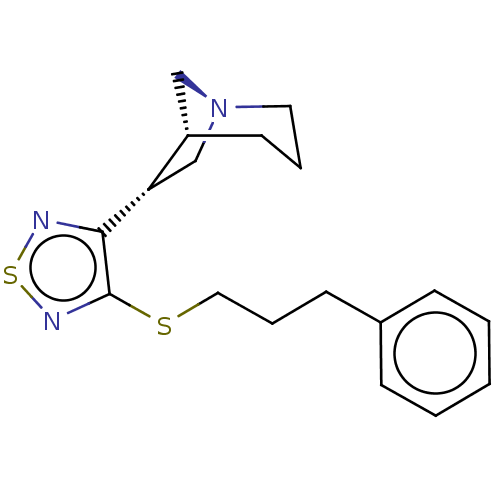
(RAT) | BDBM50072227
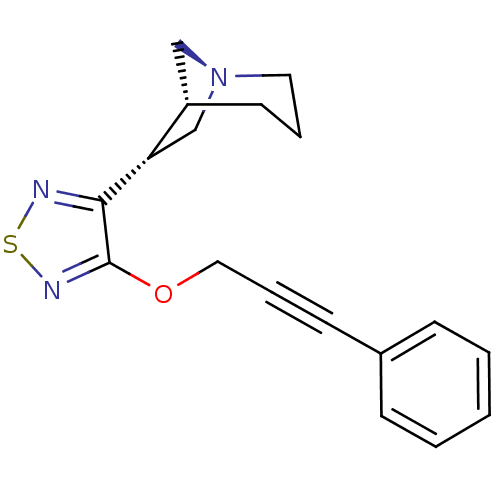
((1R,5R,6R)-6-[4-(3-Phenyl-prop-2-ynyloxy)-[1,2,5]t...)Show SMILES C(Oc1nsnc1[C@H]1C[N@]2C[C@@H]1CCC2)C#Cc1ccccc1 Show InChI InChI=1S/C18H19N3OS/c1-2-6-14(7-3-1)8-5-11-22-18-17(19-23-20-18)16-13-21-10-4-9-15(16)12-21/h1-3,6-7,15-16H,4,9-13H2/t15-,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50070735
(CHEMBL317324)Show InChI InChI=1S/C29H48N8O8/c1-4-16(3)23(27(43)34-19(5-2)28(44)45)37-26(42)21(10-8-14-33-29(31)32)36-25(41)20(9-6-7-13-30)35-24(40)18-15-17(38)11-12-22(18)39/h11-12,15-16,19-21,23,38-39H,4-10,13-14,30H2,1-3H3,(H,34,43)(H,35,40)(H,36,41)(H,37,42)(H,44,45)(H4,31,32,33)/t16?,19?,20-,21-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50070725
(CHEMBL318403)Show InChI InChI=1S/C28H43N5O8/c1-4-16(3)23(26(38)30-19(5-2)28(40)41)32-25(37)21-10-8-14-33(21)27(39)20(9-6-7-13-29)31-24(36)18-15-17(34)11-12-22(18)35/h11-12,15-16,19-21,23,34-35H,4-10,13-14,29H2,1-3H3,(H,30,38)(H,31,36)(H,32,37)(H,40,41)/t16?,19?,20-,21-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50070647
(CHEMBL329924)Show SMILES [H][C@]12C[N@](C[C@@H]1c1nsnc1SCCCc1ccccc1)CCC2 Show InChI InChI=1S/C18H23N5O/c1-3-6-13-7-4-5-8-14(13)9-15(12(2)24)23-11-22-16-17(19)20-10-21-18(16)23/h4-5,7-8,10-12,15,24H,3,6,9H2,1-2H3,(H2,19,20,21)/t12-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
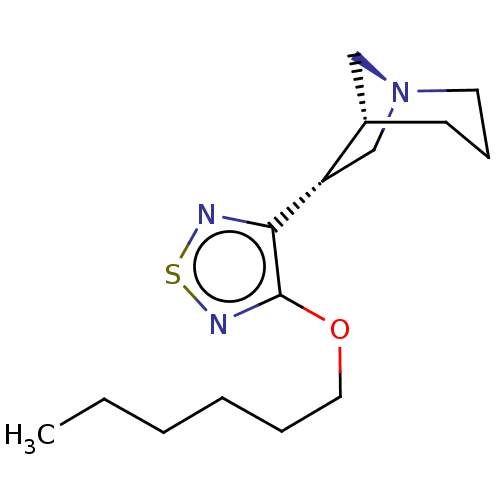
(RAT) | BDBM50213079
(CHEMBL160928)Show SMILES COc1ccc(cc1OC)C#C\C(C)=C/CO\N=C1/CN2CCC1C2 |THB:17:18:24:22.21| Show InChI InChI=1S/C20H24N2O3/c1-15(4-5-16-6-7-19(23-2)20(12-16)24-3)9-11-25-21-18-14-22-10-8-17(18)13-22/h6-7,9,12,17H,8,10-11,13-14H2,1-3H3/b15-9-,21-18+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity against muscarinic receptor using radioligand [3H]cis-methyldioxolane (CMD) binding assay in membrane preparations of rat neocortex. |
Citation and Details
BindingDB Entry DOI: 10.7270/Q23B628P |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50367705
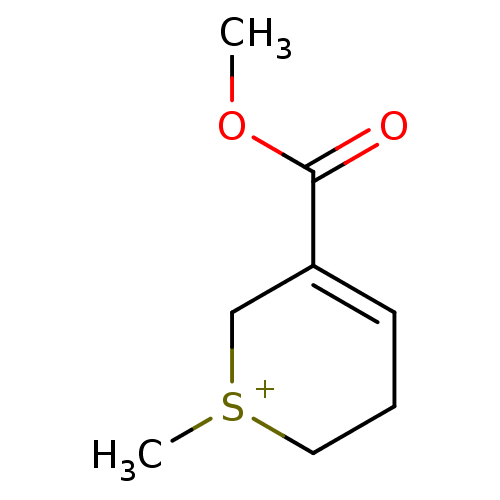
(SULFOARECOLINE IODIDE)Show InChI InChI=1S/C8H13O2S/c1-10-8(9)7-4-3-5-11(2)6-7/h4H,3,5-6H2,1-2H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Royal Danish School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of [3H]oxotremorine-M binding to rat brain membrane Muscarinic acetylcholine receptor |
J Med Chem 31: 1312-6 (1988)
BindingDB Entry DOI: 10.7270/Q2B27VW2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50003351
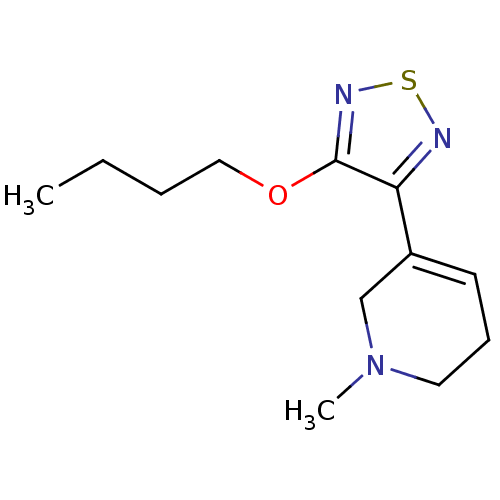
(3-(4-butoxy-1,2,5-thiadiazol-3-yl)-1-methyl-1,2,5,...)Show InChI InChI=1S/C12H19N3OS/c1-3-4-8-16-12-11(13-17-14-12)10-6-5-7-15(2)9-10/h6H,3-5,7-9H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-OXO-M (oxotremorine-M) from the central muscarinic receptor sites of the rat brain membranes |
Bioorg Med Chem Lett 2: 809-814 (1992)
Article DOI: 10.1016/S0960-894X(00)80536-3
BindingDB Entry DOI: 10.7270/Q2T72HBX |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50003351

(3-(4-butoxy-1,2,5-thiadiazol-3-yl)-1-methyl-1,2,5,...)Show InChI InChI=1S/C12H19N3OS/c1-3-4-8-16-12-11(13-17-14-12)10-6-5-7-15(2)9-10/h6H,3-5,7-9H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50004665

((oxotremorine)1-(4-Pyrrolidin-1-yl-but-2-ynyl)-pyr...)Show InChI InChI=1S/C12H18N2O/c15-12-6-5-11-14(12)10-4-3-9-13-7-1-2-8-13/h1-2,5-11H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of 0.1 nM [3H]cis-methyldioxolane binding to rat neocortex muscarinic receptor |
Bioorg Med Chem Lett 2: 803-808 (1992)
Article DOI: 10.1016/S0960-894X(00)80535-1
BindingDB Entry DOI: 10.7270/Q2Z0382N |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50003369
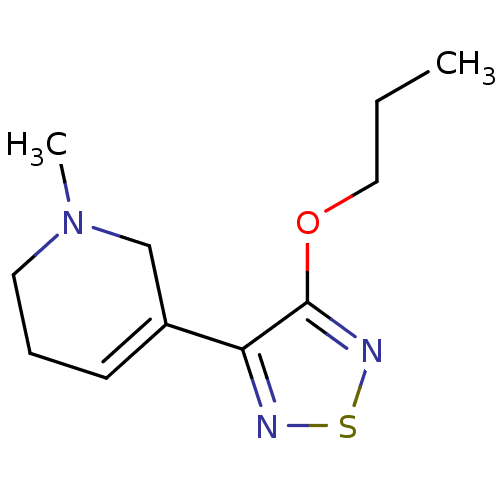
(1-Methyl-5-(4-propoxy-[1,2,5]thiadiazol-3-yl)-1,2,...)Show InChI InChI=1S/C11H17N3OS/c1-3-7-15-11-10(12-16-13-11)9-5-4-6-14(2)8-9/h5H,3-4,6-8H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50213071
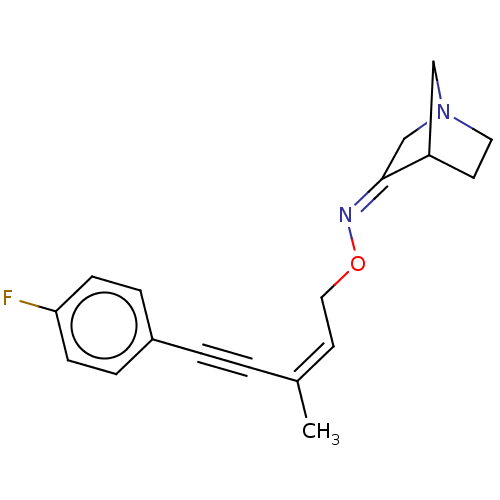
(CHEMBL160939)Show SMILES C\C(=C\CO\N=C1\CN2CCC1C2)C#Cc1ccc(F)cc1 |THB:5:6:12:10.9| Show InChI InChI=1S/C18H19FN2O/c1-14(2-3-15-4-6-17(19)7-5-15)9-11-22-20-18-13-21-10-8-16(18)12-21/h4-7,9,16H,8,10-13H2,1H3/b14-9-,20-18- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity against muscarinic receptor using radioligand [3H]cis-methyldioxolane (CMD) binding assay in membrane preparations of rat neocortex. |
Citation and Details
BindingDB Entry DOI: 10.7270/Q23B628P |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50223182
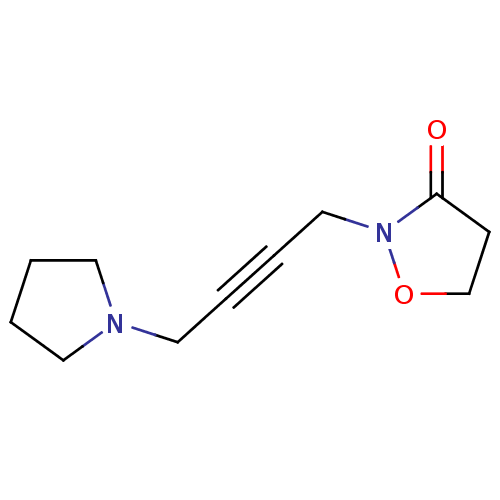
(2-(4-(pyrrolidin-1-yl)but-2-ynyl)isoxazolidin-3-on...)Show InChI InChI=1S/C11H16N2O2/c14-11-5-10-15-13(11)9-4-3-8-12-6-1-2-7-12/h1-2,5-10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic receptor M2 in rat brain using [3H]-QNB (quinuclidinyl benzylate) radioligand at a concentration of 0.12 nM |
Bioorg Med Chem Lett 7: 1033-1036 (1997)
Article DOI: 10.1016/S0960-894X(97)00150-9
BindingDB Entry DOI: 10.7270/Q2S46RZ2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
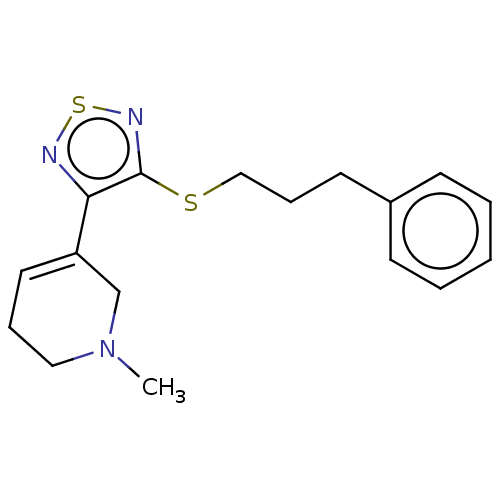
(RAT) | BDBM50072225

((1R,5R,6R)-6-[4-(3-Phenyl-prop-2-ynylsulfanyl)-[1,...)Show SMILES C(Sc1nsnc1[C@H]1C[N@]2C[C@@H]1CCC2)C#Cc1ccccc1 Show InChI InChI=1S/C18H19N3S2/c1-2-6-14(7-3-1)8-5-11-22-18-17(19-23-20-18)16-13-21-10-4-9-15(16)12-21/h1-3,6-7,15-16H,4,9-13H2/t15-,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50070684

(CHEMBL321073)Show SMILES [H][C@@]12CC[N@@](C[C@@H]1c1nsnc1SCCCc1ccccc1)C2 Show InChI InChI=1S/C28H43N7O8/c1-4-15(3)22(25(40)32-18(5-2)27(42)43)34-24(39)20-9-7-13-35(20)26(41)19(8-6-12-31-28(29)30)33-23(38)17-14-16(36)10-11-21(17)37/h10-11,14-15,18-20,22,36-37H,4-9,12-13H2,1-3H3,(H,32,40)(H,33,38)(H,34,39)(H,42,43)(H4,29,30,31)/t15?,18?,19-,20-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM46858
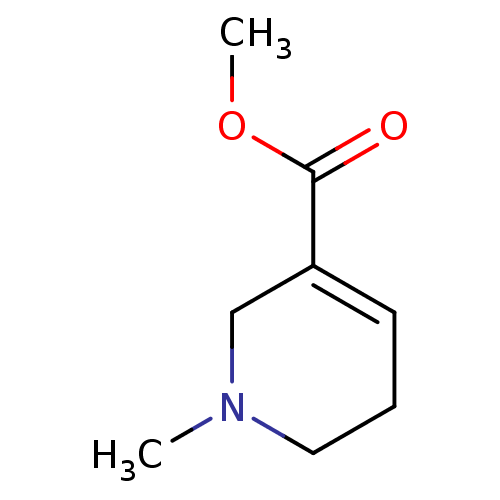
(1-methyl-3,6-dihydro-2H-pyridine-5-carboxylic acid...)Show InChI InChI=1S/C8H13NO2/c1-9-5-3-4-7(6-9)8(10)11-2/h4H,3,5-6H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Royal Danish School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of [3H]oxotremorine-M binding to rat brain membrane Muscarinic acetylcholine receptor |
J Med Chem 31: 1312-6 (1988)
BindingDB Entry DOI: 10.7270/Q2B27VW2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50002338
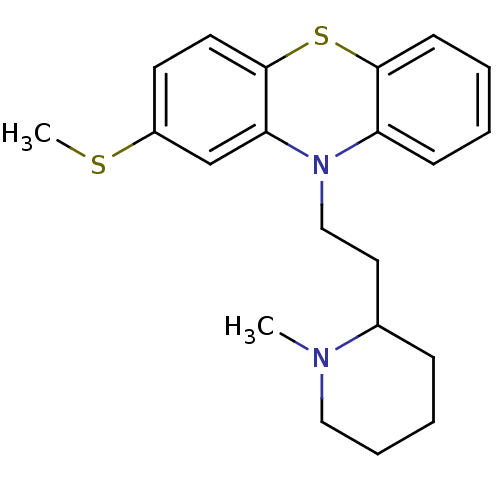
((Thioridazine)10-[2-(1-Methyl-piperidin-2-yl)-ethy...)Show InChI InChI=1S/C21H26N2S2/c1-22-13-6-5-7-16(22)12-14-23-18-8-3-4-9-20(18)25-21-11-10-17(24-2)15-19(21)23/h3-4,8-11,15-16H,5-7,12-14H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Displacement of [3H]quinuclidinyl benzilate (QNB) from Muscarinic acetylcholine receptor in rat brain |
J Med Chem 31: 454-61 (1988)
BindingDB Entry DOI: 10.7270/Q25T3JGG |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50004665

((oxotremorine)1-(4-Pyrrolidin-1-yl-but-2-ynyl)-pyr...)Show InChI InChI=1S/C12H18N2O/c15-12-6-5-11-14(12)10-4-3-9-13-7-1-2-8-13/h1-2,5-11H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic receptor M2 in rat brain using [3H]-QNB (quinuclidinyl benzylate) radioligand at a concentration of 0.12 nM |
Bioorg Med Chem Lett 7: 1033-1036 (1997)
Article DOI: 10.1016/S0960-894X(97)00150-9
BindingDB Entry DOI: 10.7270/Q2S46RZ2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50003366
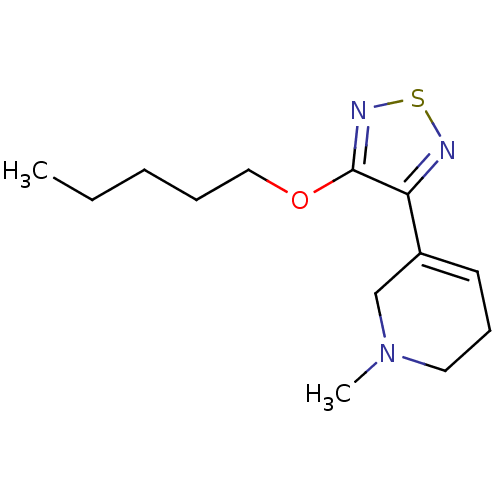
(1-Methyl-5-(4-pentyloxy-[1,2,5]thiadiazol-3-yl)-1,...)Show InChI InChI=1S/C13H21N3OS/c1-3-4-5-9-17-13-12(14-18-15-13)11-7-6-8-16(2)10-11/h7H,3-6,8-10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligand |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50212901
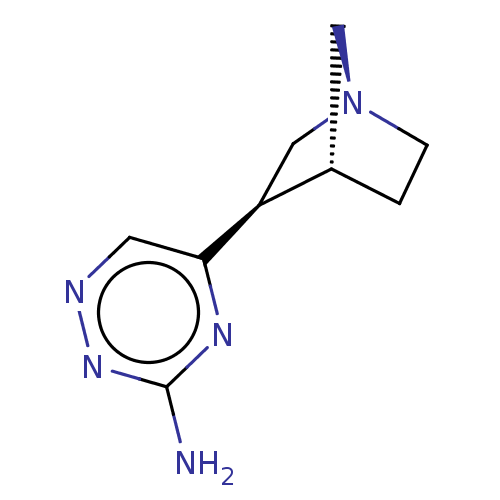
(CHEMBL280748)Show InChI InChI=1S/C9H13N5/c10-9-12-8(3-11-13-9)7-5-14-2-1-6(7)4-14/h3,6-7H,1-2,4-5H2,(H2,10,12,13)/t6-,7-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Ability to inhibit [3H]oxotremorine-M (OXO-M) binding in rat cerebral cortex |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2NG4SSP |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229676

(CHEMBL78697)Show InChI InChI=1S/C7H12N6/c8-7-9-11-13(10-7)6-4-12-2-1-5(6)3-12/h5-6H,1-4H2,(H2,8,10)/t5-,6-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229669
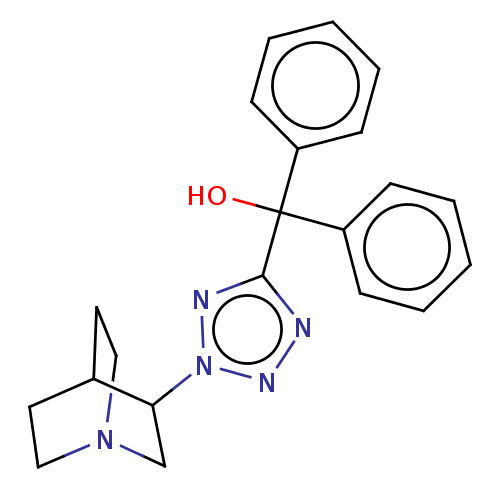
(CHEMBL309432)Show SMILES OC(c1nnn(n1)C1CN2CCC1CC2)(c1ccccc1)c1ccccc1 |(9.08,-4.44,;7.54,-4.44,;6.01,-4.28,;5.24,-2.95,;3.73,-3.27,;3.57,-4.81,;4.97,-5.43,;2.22,-5.58,;2.22,-7.12,;.9,-7.89,;-.44,-7.12,;-.44,-5.58,;.9,-4.81,;1.58,-6.16,;.11,-6.56,;8.45,-3.21,;7.81,-1.81,;8.71,-.56,;10.25,-.72,;10.88,-2.13,;9.98,-3.37,;8.16,-5.85,;7.26,-7.1,;7.87,-8.5,;9.41,-8.66,;10.31,-7.41,;9.69,-6.01,)| Show InChI InChI=1S/C21H23N5O/c27-21(17-7-3-1-4-8-17,18-9-5-2-6-10-18)20-22-24-26(23-20)19-15-25-13-11-16(19)12-14-25/h1-10,16,19,27H,11-15H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro displacement of [3H]quinuclidinyl benzilate (QNB) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50004665

((oxotremorine)1-(4-Pyrrolidin-1-yl-but-2-ynyl)-pyr...)Show InChI InChI=1S/C12H18N2O/c15-12-6-5-11-14(12)10-4-3-9-13-7-1-2-8-13/h1-2,5-11H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Sandoz Pharma Ltd.
Curated by ChEMBL
| Assay Description
Ability to displace [3H]cis--2-methyl-5-((dimethylamino)methyl)-1,3-di oxolane from muscarinic acetylcholine receptor in rat cortical tissue. |
J Med Chem 35: 15-27 (1992)
BindingDB Entry DOI: 10.7270/Q29022RV |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229528

(CHEMBL93406)Show InChI InChI=1S/C9H13N3O/c1-6-10-9(13-11-6)8-5-12-3-2-7(8)4-12/h7-8H,2-5H2,1H3/t7-,8-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
The compound was tested in vitro for central muscarinic acetylcholine receptor affinity to displace [3H]oxotremorine-M from rat cerebral cortex; Valu... |
J Med Chem 34: 2726-35 (1991)
BindingDB Entry DOI: 10.7270/Q2BG2R7J |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50453074
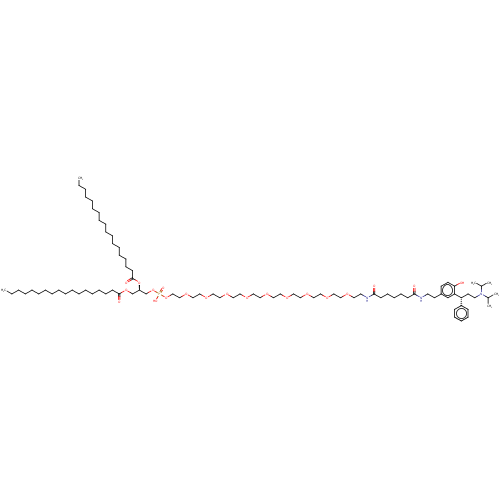
(CHEMBL4215958)Show SMILES CCCCCCCCCCCCCCCCCC(=O)OC[C@H](COP(O)(=O)OCCOCCOCCOCCOCCOCCOCCOCCOCCOCCNC(=O)CCCCCC(=O)NCCc1ccc(O)c(c1)[C@H](CCN(C(C)C)C(C)C)c1ccccc1)OC(=O)CCCCCCCCCCCCCCCCC |r| Show InChI InChI=1S/C89H160N3O20P/c1-7-9-11-13-15-17-19-21-23-25-27-29-31-33-41-47-88(96)109-76-82(112-89(97)48-42-34-32-30-28-26-24-22-20-18-16-14-12-10-8-2)77-111-113(98,99)110-74-73-108-72-71-107-70-69-106-68-67-105-66-65-104-64-63-103-62-61-102-60-59-101-58-57-100-56-54-91-87(95)46-40-36-39-45-86(94)90-53-51-80-49-50-85(93)84(75-80)83(81-43-37-35-38-44-81)52-55-92(78(3)4)79(5)6/h35,37-38,43-44,49-50,75,78-79,82-83,93H,7-34,36,39-42,45-48,51-74,76-77H2,1-6H3,(H,90,94)(H,91,95)(H,98,99)/t82-,83-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from muscarinic receptor in Sprague-Dawley rat brain homogenates after 60 mins by liquid scintillation counting method |
J Med Chem 61: 4020-4029 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00041
BindingDB Entry DOI: 10.7270/Q29Z97H3 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50453077
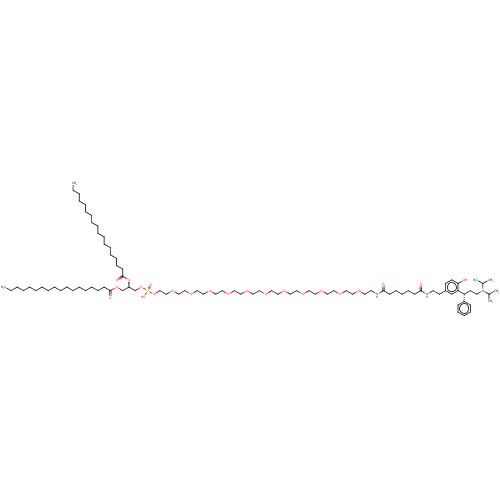
(CHEMBL4216478)Show SMILES CCCCCCCCCCCCCCCCCC(=O)OC[C@H](COP(O)(=O)OCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCOCCNC(=O)CCCCCC(=O)NCCc1ccc(O)c(c1)[C@H](CCN(C(C)C)C(C)C)c1ccccc1)OC(=O)CCCCCCCCCCCCCCCCC |r| Show InChI InChI=1S/C93H168N3O22P/c1-7-9-11-13-15-17-19-21-23-25-27-29-31-33-41-47-92(100)115-80-86(118-93(101)48-42-34-32-30-28-26-24-22-20-18-16-14-12-10-8-2)81-117-119(102,103)116-78-77-114-76-75-113-74-73-112-72-71-111-70-69-110-68-67-109-66-65-108-64-63-107-62-61-106-60-59-105-58-57-104-56-54-95-91(99)46-40-36-39-45-90(98)94-53-51-84-49-50-89(97)88(79-84)87(85-43-37-35-38-44-85)52-55-96(82(3)4)83(5)6/h35,37-38,43-44,49-50,79,82-83,86-87,97H,7-34,36,39-42,45-48,51-78,80-81H2,1-6H3,(H,94,98)(H,95,99)(H,102,103)/t86-,87-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Shizuoka
Curated by ChEMBL
| Assay Description
Displacement of [3H]NMS from muscarinic receptor in Sprague-Dawley rat brain homogenates after 60 mins by liquid scintillation counting method |
J Med Chem 61: 4020-4029 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00041
BindingDB Entry DOI: 10.7270/Q29Z97H3 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50023702
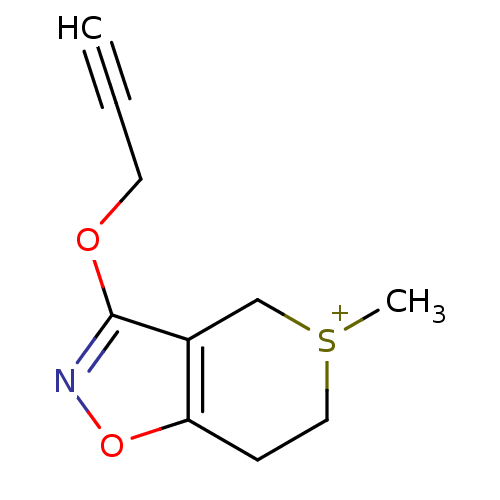
(5-Methyl-3-prop-2-ynyloxy-6,7-dihydro-4H-thiopyran...)Show InChI InChI=1S/C10H12NO2S/c1-3-5-12-10-8-7-14(2)6-4-9(8)13-11-10/h1H,4-7H2,2H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Royal Danish School of Pharmacy
Curated by ChEMBL
| Assay Description
Inhibition of [3H]oxotremorine-M binding to rat brain membrane Muscarinic acetylcholine receptor |
J Med Chem 31: 1312-6 (1988)
BindingDB Entry DOI: 10.7270/Q2B27VW2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50072227

((1R,5R,6R)-6-[4-(3-Phenyl-prop-2-ynyloxy)-[1,2,5]t...)Show SMILES C(Oc1nsnc1[C@H]1C[N@]2C[C@@H]1CCC2)C#Cc1ccccc1 Show InChI InChI=1S/C18H19N3OS/c1-2-6-14(7-3-1)8-5-11-22-18-17(19-23-20-18)16-13-21-10-4-9-15(16)12-21/h1-3,6-7,15-16H,4,9-13H2/t15-,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S
Curated by ChEMBL
| Assay Description
Stimulation of cAMP in CHO cells expressing human m2 receptor |
Bioorg Med Chem Lett 8: 2897-902 (1999)
BindingDB Entry DOI: 10.7270/Q27M08GK |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229684
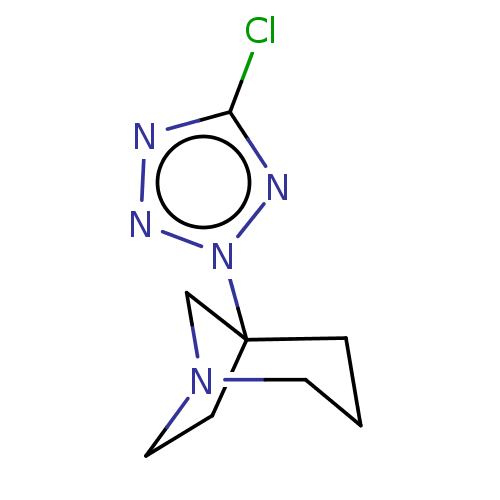
(CHEMBL78615)Show InChI InChI=1S/C8H12ClN5/c9-7-10-12-14(11-7)8-2-1-4-13(6-8)5-3-8/h1-6H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic acetylcholine receptor |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
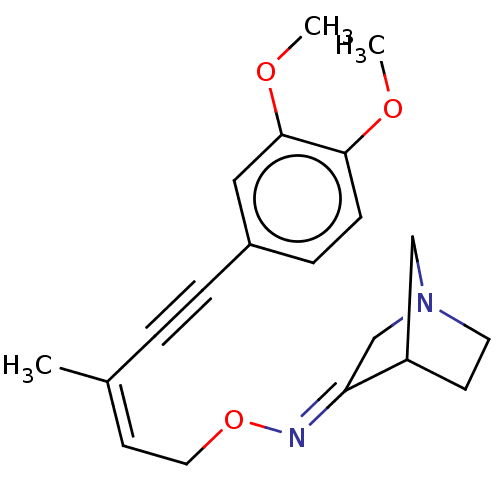
(RAT) | BDBM50065213
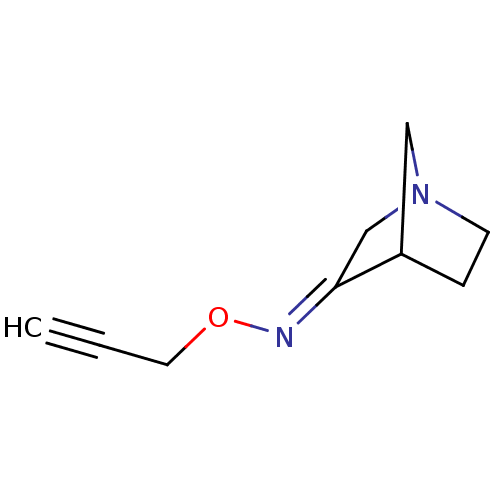
(1-Aza-bicyclo[2.2.1]heptan-3-one O-prop-2-ynyl-oxi...)Show InChI InChI=1S/C9H12N2O/c1-2-5-12-10-9-7-11-4-3-8(9)6-11/h1,8H,3-7H2/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of [3H]cis-methyldioxolane binding to label agonist sites (RCMD) in rat neocortex |
Bioorg Med Chem Lett 2: 821-826 (1992)
Article DOI: 10.1016/S0960-894X(00)80538-7
BindingDB Entry DOI: 10.7270/Q2PG1RNN |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50280560
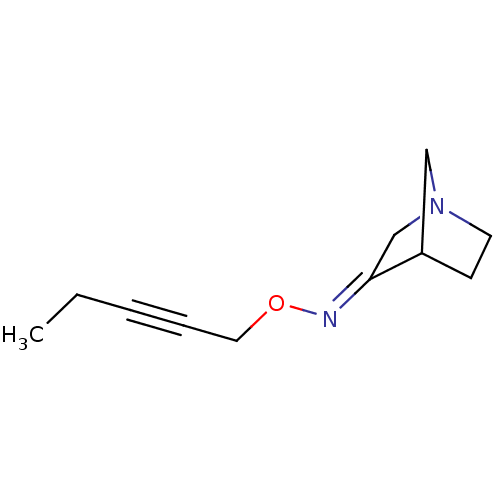
(1-Aza-bicyclo[2.2.1]heptan-3-one O-pent-2-ynyl-oxi...)Show InChI InChI=1S/C11H16N2O/c1-2-3-4-7-14-12-11-9-13-6-5-10(11)8-13/h10H,2,5-9H2,1H3/b12-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of [3H]cis-methyldioxolane binding to label agonist sites (RCMD) in rat neocortex |
Bioorg Med Chem Lett 2: 821-826 (1992)
Article DOI: 10.1016/S0960-894X(00)80538-7
BindingDB Entry DOI: 10.7270/Q2PG1RNN |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229669

(CHEMBL309432)Show SMILES OC(c1nnn(n1)C1CN2CCC1CC2)(c1ccccc1)c1ccccc1 |(9.08,-4.44,;7.54,-4.44,;6.01,-4.28,;5.24,-2.95,;3.73,-3.27,;3.57,-4.81,;4.97,-5.43,;2.22,-5.58,;2.22,-7.12,;.9,-7.89,;-.44,-7.12,;-.44,-5.58,;.9,-4.81,;1.58,-6.16,;.11,-6.56,;8.45,-3.21,;7.81,-1.81,;8.71,-.56,;10.25,-.72,;10.88,-2.13,;9.98,-3.37,;8.16,-5.85,;7.26,-7.1,;7.87,-8.5,;9.41,-8.66,;10.31,-7.41,;9.69,-6.01,)| Show InChI InChI=1S/C21H23N5O/c27-21(17-7-3-1-4-8-17,18-9-5-2-6-10-18)20-22-24-26(23-20)19-15-25-13-11-16(19)12-14-25/h1-10,16,19,27H,11-15H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50065221
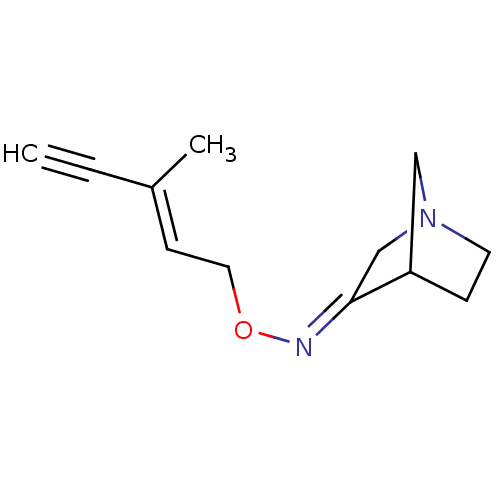
(1-Aza-bicyclo[2.2.1]heptan-3-one O-((E)-3-methyl-p...)Show InChI InChI=1S/C12H16N2O/c1-3-10(2)5-7-15-13-12-9-14-6-4-11(12)8-14/h1,5,11H,4,6-9H2,2H3/b10-5+,13-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity against muscarinic receptor using radioligand [3H]cis-methyldioxolane (CMD) binding assay in membrane preparations of rat neocortex. |
Citation and Details
BindingDB Entry DOI: 10.7270/Q23B628P |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50452855

(Isoptpo Hyoscine | Scopolamine)Show SMILES [H][C@@]12O[C@@]1([H])[C@]1([H])C[C@@H](C[C@@]2([H])N1C)OC(=O)[C@H](CO)c1ccccc1 |r,TLB:14:8:12:1.3,2:1:12:8.7.9,2:3:12:8.7.9| Show InChI InChI=1S/C17H21NO4/c1-18-13-7-11(8-14(18)16-15(13)22-16)21-17(20)12(9-19)10-5-3-2-4-6-10/h2-6,11-16,19H,7-9H2,1H3/t11-,12-,13-,14+,15+,16+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]quinuclidinyl benzilate (QNB) binding from rat forebrain membranes in the presence of Zn |
J Med Chem 39: 570-81 (1996)
Article DOI: 10.1021/jm9506433
BindingDB Entry DOI: 10.7270/Q2BR8R7K |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50452855

(Isoptpo Hyoscine | Scopolamine)Show SMILES [H][C@@]12O[C@@]1([H])[C@]1([H])C[C@@H](C[C@@]2([H])N1C)OC(=O)[C@H](CO)c1ccccc1 |r,TLB:14:8:12:1.3,2:1:12:8.7.9,2:3:12:8.7.9| Show InChI InChI=1S/C17H21NO4/c1-18-13-7-11(8-14(18)16-15(13)22-16)21-17(20)12(9-19)10-5-3-2-4-6-10/h2-6,11-16,19H,7-9H2,1H3/t11-,12-,13-,14+,15+,16+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of [3H]quinuclidinyl benzilate (QNB) binding from rat forebrain membranes in the absence of Zn |
J Med Chem 39: 570-81 (1996)
Article DOI: 10.1021/jm9506433
BindingDB Entry DOI: 10.7270/Q2BR8R7K |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50291550

(3-(4-Pyrrolidin-1-yl-but-2-ynyloxy)-4,5-dihydro-is...)Show InChI InChI=1S/C11H16N2O2/c1-2-7-13(6-1)8-3-4-9-14-11-5-10-15-12-11/h1-2,5-10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was assessed for the binding affinity against muscarinic receptor subtypes in rat brain using [3H]OXO-M radioligand as a muscarinic receptor... |
Bioorg Med Chem Lett 7: 1033-1036 (1997)
Article DOI: 10.1016/S0960-894X(97)00150-9
BindingDB Entry DOI: 10.7270/Q2S46RZ2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data