

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50213779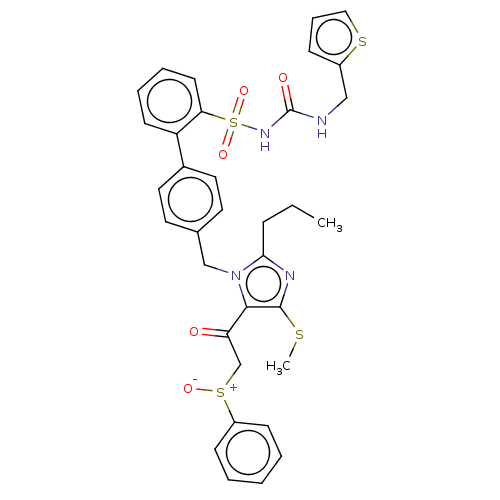 (CHEMBL313598) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Concentration required for 50% inhibition of [125I]- AII binding to rat liver membrane preparation (AT1) | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC63CR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50213551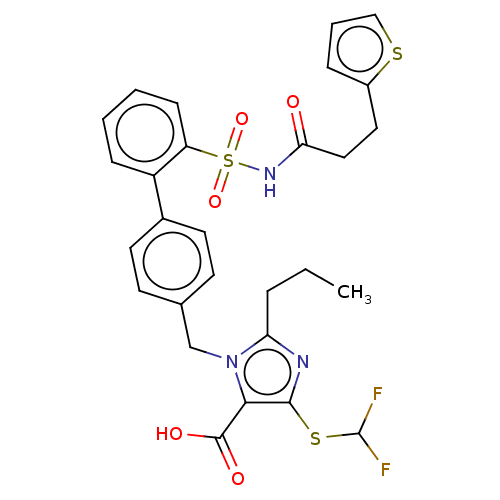 (CHEMBL90618) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description In vitro displacement of [125I]angiotensin II from rat liver (Angiotensin 1 receptor) membrane preparation | Citation and Details BindingDB Entry DOI: 10.7270/Q2S184PR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50213695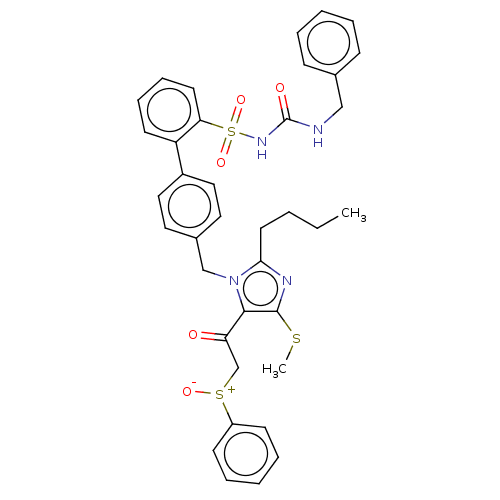 (CHEMBL89032) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Concentration required for 50% inhibition of [125I]- AII binding to rat liver membrane preparation (AT1) | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC63CR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50213592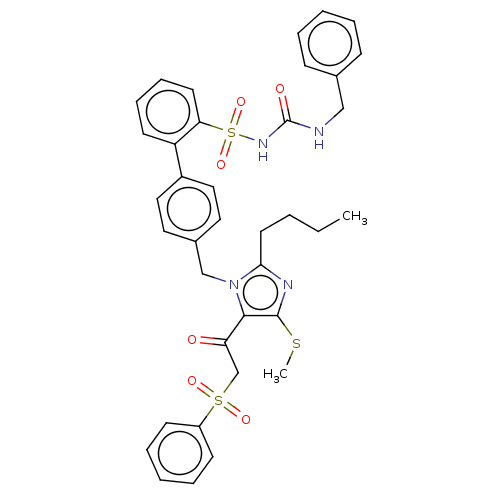 (CHEMBL315236) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Concentration required for 50% inhibition of [125I]- AII binding to rat liver membrane preparation (AT1) | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC63CR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50213685 (CHEMBL431853) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Concentration required for 50% inhibition of [125I]- AII binding to rat liver membrane preparation (AT1) | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC63CR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50213561 (CHEMBL312937) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description In vitro displacement of [125I]angiotensin II from rat liver (Angiotensin 1 receptor) membrane preparation | Citation and Details BindingDB Entry DOI: 10.7270/Q2S184PR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50213505 (CHEMBL314975) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description In vitro displacement of [125I]angiotensin II from rat liver (Angiotensin 1 receptor) membrane preparation | Citation and Details BindingDB Entry DOI: 10.7270/Q2S184PR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50213687 (CHEMBL87244) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Concentration required for 50% inhibition of [125I]- AII binding to rat liver membrane preparation (AT1) | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC63CR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50213697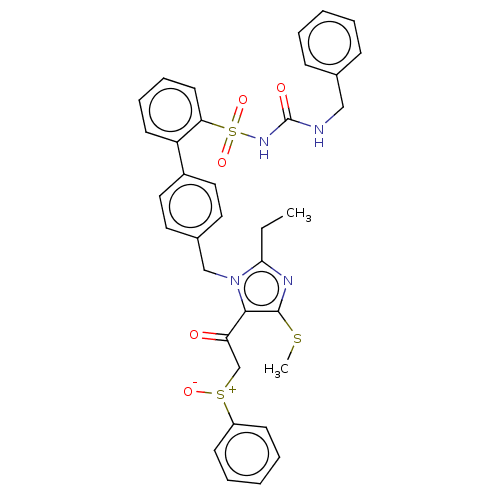 (CHEMBL84908) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Concentration required for 50% inhibition of [125I]- AII binding to rat liver membrane preparation (AT1) | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC63CR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50213687 (CHEMBL87244) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Concentration required for 50% inhibition of [125I]- AII binding to rat liver membrane preparation (AT1) | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC63CR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50213782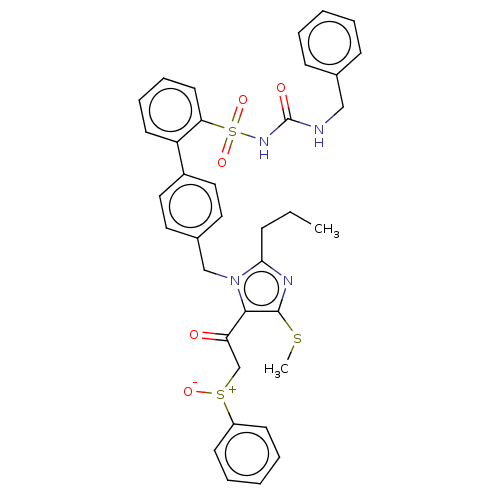 (CHEMBL264304) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Concentration required for 50% inhibition of [125I]- AII binding to rat liver membrane preparation (AT1) | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC63CR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50213688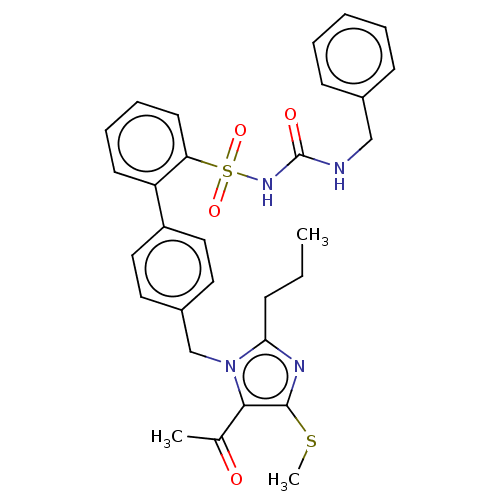 (CHEMBL86538) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Concentration required for 50% inhibition of [125I]- AII binding to rat liver membrane preparation (AT1) | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC63CR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50213593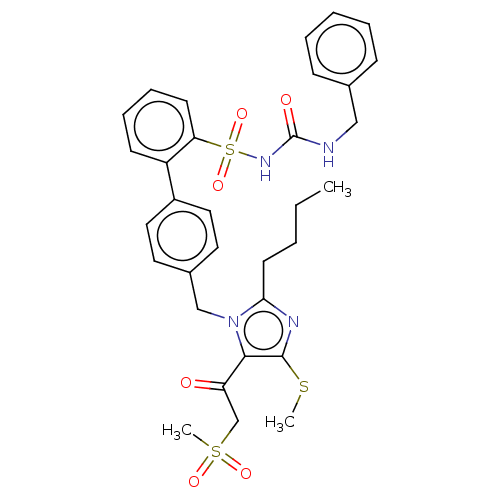 (CHEMBL91046) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Concentration required for 50% inhibition of [125I]- AII binding to rat liver membrane preparation (AT1) | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC63CR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470233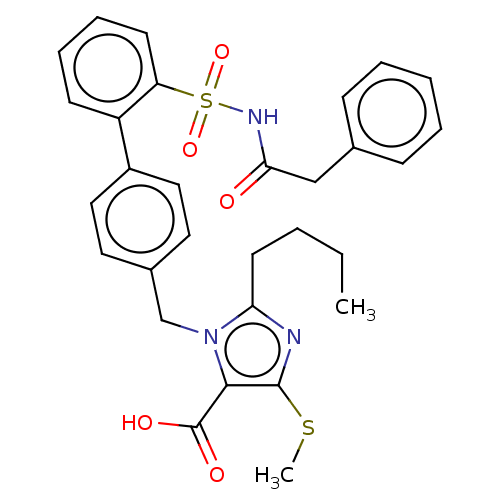 (CHEMBL80177) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470229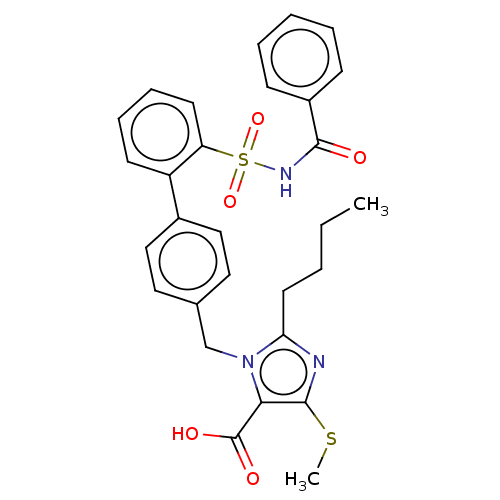 (CHEMBL76870) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50213681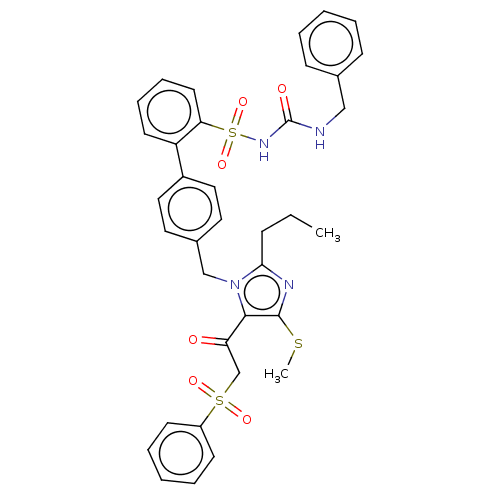 (CHEMBL433010) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Concentration required for 50% inhibition of [125I]- AII binding to rat liver membrane preparation (AT1) | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC63CR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50213680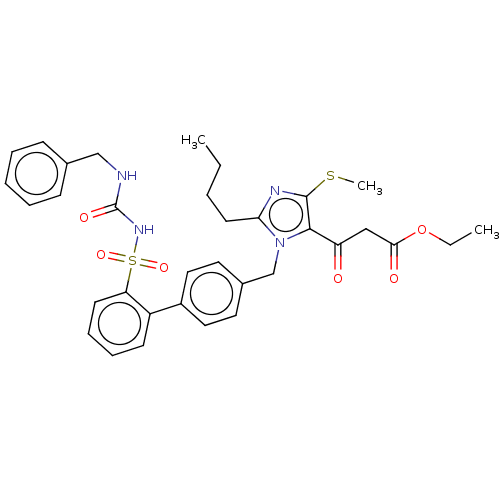 (CHEMBL87091) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Concentration required for 50% inhibition of [125I]- AII binding to rat liver membrane preparation (AT1) | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC63CR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50213783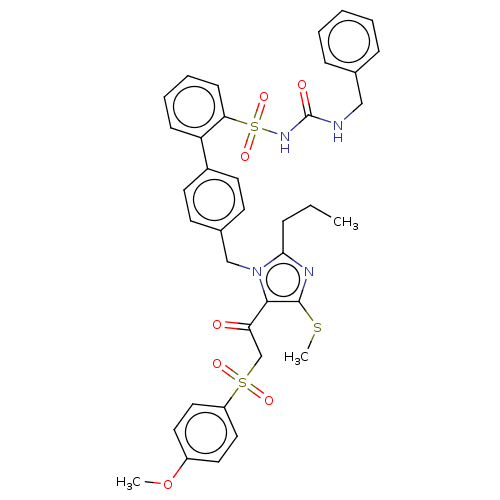 (CHEMBL328985) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Concentration required for 50% inhibition of [125I]- AII binding to rat liver membrane preparation (AT1) | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC63CR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470236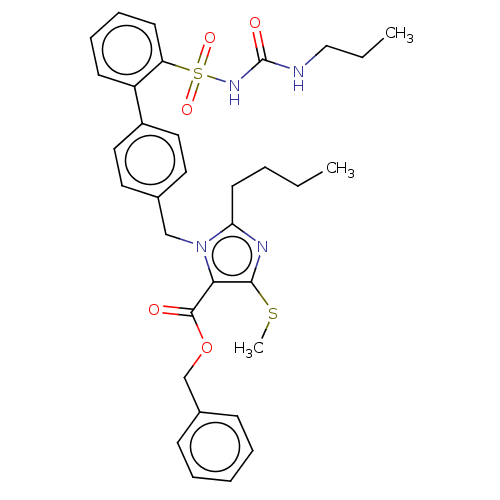 (CHEMBL309313) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470239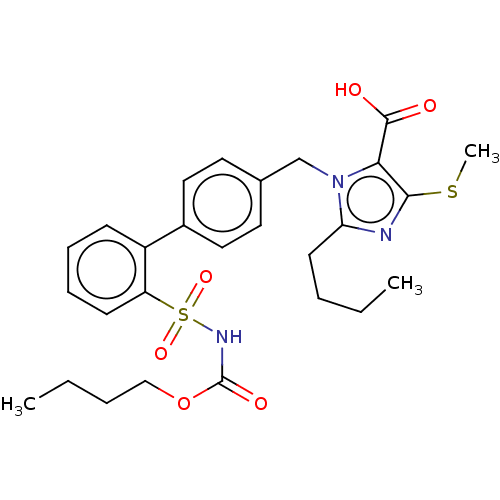 (CHEMBL311312) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50213504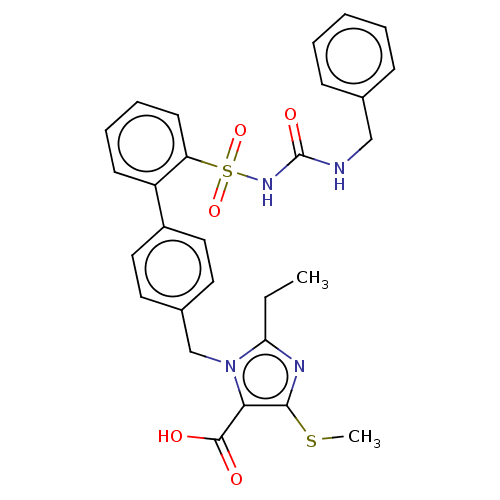 (CHEMBL89369) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description In vitro displacement of [125I]angiotensin II from rat liver (Angiotensin 1 receptor) membrane preparation | Citation and Details BindingDB Entry DOI: 10.7270/Q2S184PR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50213457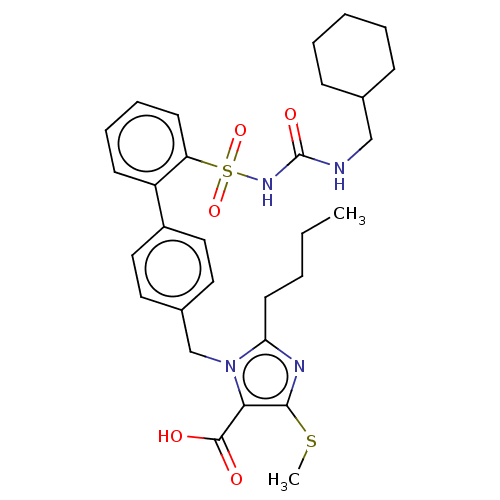 (CHEMBL84686) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description In vitro displacement of [125I]angiotensin II from rat liver (Angiotensin 1 receptor) membrane preparation | Citation and Details BindingDB Entry DOI: 10.7270/Q2S184PR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50213500 (CHEMBL86337) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description In vitro displacement of [125I]angiotensin II from rat liver (Angiotensin 1 receptor) membrane preparation | Citation and Details BindingDB Entry DOI: 10.7270/Q2S184PR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50213591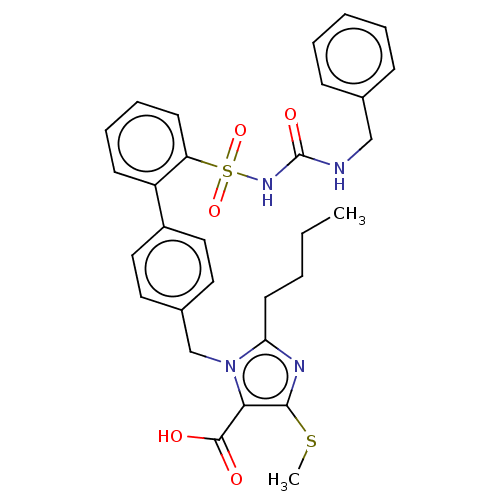 (CHEMBL84795) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Concentration required for 50% inhibition of [125I]- AII binding to rat liver membrane preparation (AT1) | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC63CR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50213552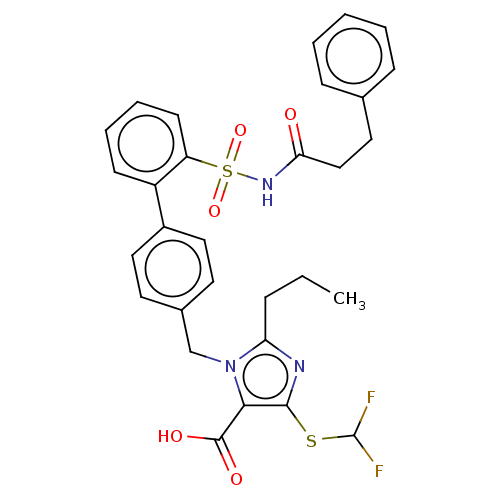 (CHEMBL328302) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description In vitro displacement of [125I]angiotensin II from rat liver (Angiotensin 1 receptor) membrane preparation | Citation and Details BindingDB Entry DOI: 10.7270/Q2S184PR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50213567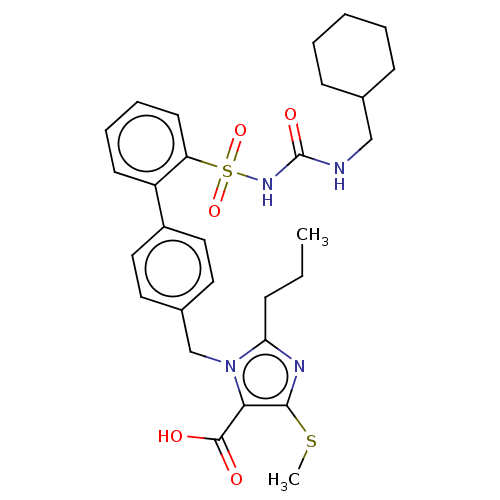 (CHEMBL315983) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description In vitro displacement of [125I]angiotensin II from rat liver (Angiotensin 1 receptor) membrane preparation | Citation and Details BindingDB Entry DOI: 10.7270/Q2S184PR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50213460 (CHEMBL85081) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description In vitro displacement of [125I]angiotensin II from rat liver (Angiotensin 1 receptor) membrane preparation | Citation and Details BindingDB Entry DOI: 10.7270/Q2S184PR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50213591 (CHEMBL84795) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description In vitro displacement of [125I]angiotensin II from rat liver (Angiotensin 1 receptor) membrane preparation | Citation and Details BindingDB Entry DOI: 10.7270/Q2S184PR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50213458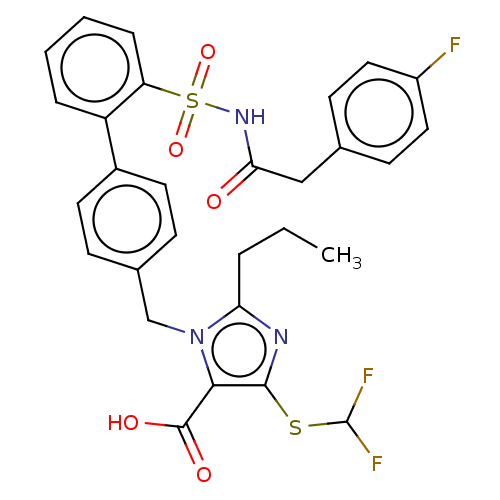 (CHEMBL90672) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description In vitro displacement of [125I]angiotensin II from rat liver (Angiotensin 1 receptor) membrane preparation | Citation and Details BindingDB Entry DOI: 10.7270/Q2S184PR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50213456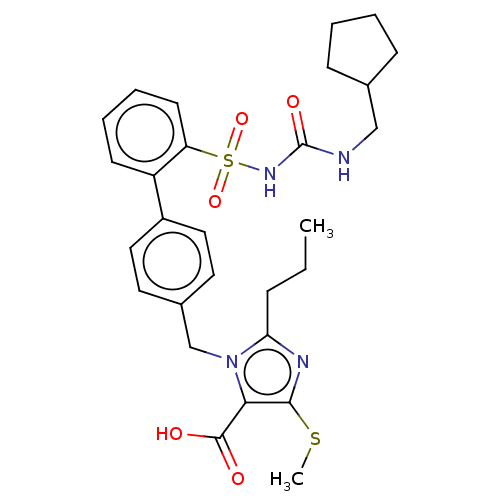 (CHEMBL315000) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description In vitro displacement of [125I]angiotensin II from rat liver (Angiotensin 1 receptor) membrane preparation | Citation and Details BindingDB Entry DOI: 10.7270/Q2S184PR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50283305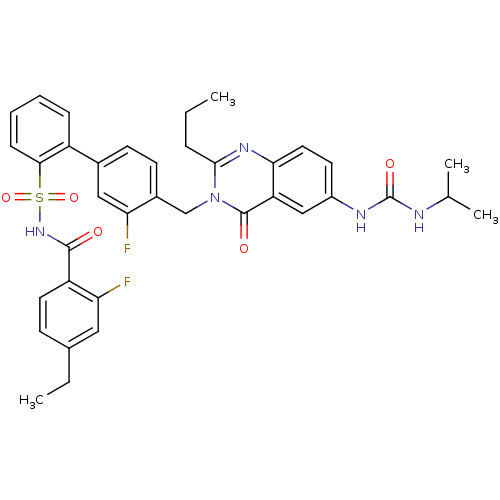 (3'-Fluoro-4'-[6-(3-isopropyl-ureido)-4-oxo-2-propy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vivo inhibitory concentration against Angiotensin II receptor, type 1 of rat adrenal membrane | Bioorg Med Chem Lett 4: 2337-2342 (1994) Article DOI: 10.1016/0960-894X(94)85036-4 BindingDB Entry DOI: 10.7270/Q2X066Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50283317 (2-Propyl-3-(3-fluoro-2'-(3-methylbutyloxycarbonyla...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vivo inhibitory concentration of the compound against Angiotensin II, type 2 of rat midbrain membrane | Bioorg Med Chem Lett 4: 2337-2342 (1994) Article DOI: 10.1016/0960-894X(94)85036-4 BindingDB Entry DOI: 10.7270/Q2X066Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50283300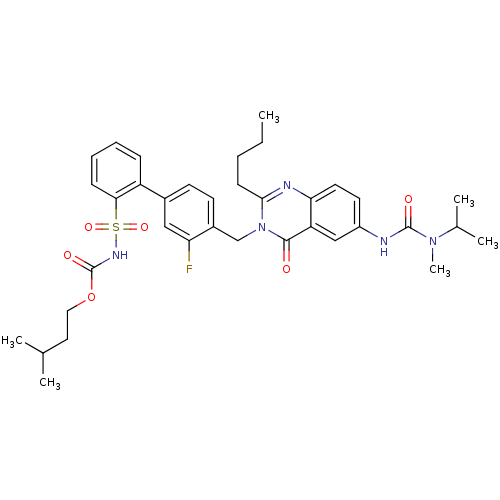 (2-Butyl-3-(3-fluoro-2'-(3-methylbutyloxycarbonylam...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vivo inhibitory concentration of the compound against Angiotensin II, type 2 of rat midbrain membrane | Bioorg Med Chem Lett 4: 2337-2342 (1994) Article DOI: 10.1016/0960-894X(94)85036-4 BindingDB Entry DOI: 10.7270/Q2X066Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50283310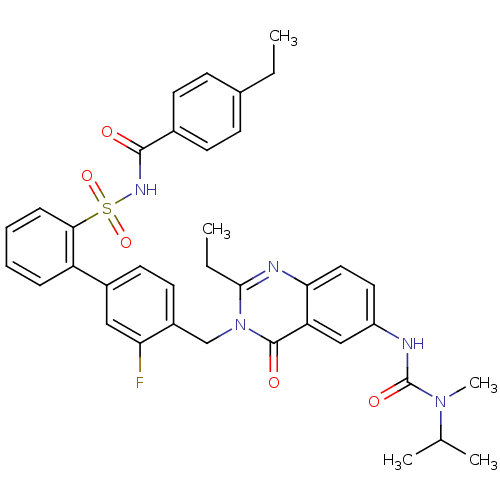 (4'-[2-Ethyl-6-(3-isopropyl-3-methyl-ureido)-4-oxo-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vivo inhibitory concentration of the compound against Angiotensin II, type 2 of rat midbrain membrane | Bioorg Med Chem Lett 4: 2337-2342 (1994) Article DOI: 10.1016/0960-894X(94)85036-4 BindingDB Entry DOI: 10.7270/Q2X066Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50283312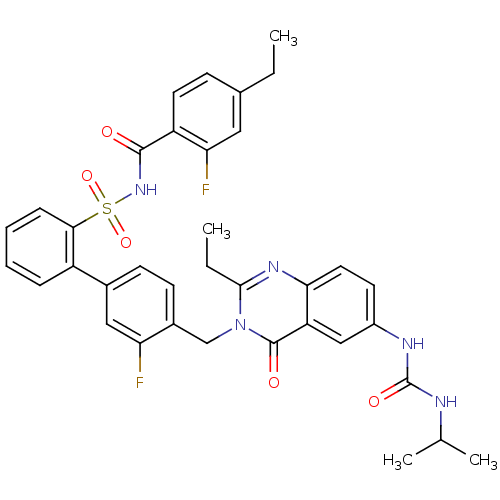 (4'-[2-Ethyl-6-(3-isopropyl-ureido)-4-oxo-4H-quinaz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vivo inhibitory concentration of the compound against AT2 receptor of rat adrenal membrane | Bioorg Med Chem Lett 4: 2337-2342 (1994) Article DOI: 10.1016/0960-894X(94)85036-4 BindingDB Entry DOI: 10.7270/Q2X066Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50213499 (CHEMBL88653) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description In vitro displacement of [125I]angiotensin II from rat liver (Angiotensin 1 receptor) membrane preparation | Citation and Details BindingDB Entry DOI: 10.7270/Q2S184PR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50283316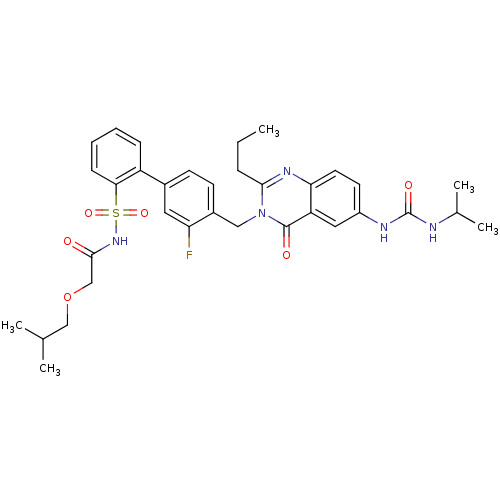 (3'-Fluoro-4'-[6-(3-isopropyl-ureido)-4-oxo-2-propy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vivo inhibitory concentration of the compound against Angiotensin II, type 2 of rat midbrain membrane | Bioorg Med Chem Lett 4: 2337-2342 (1994) Article DOI: 10.1016/0960-894X(94)85036-4 BindingDB Entry DOI: 10.7270/Q2X066Z9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50067536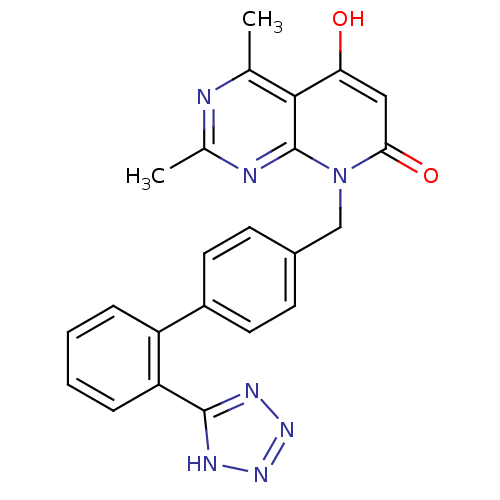 (5-Hydroxy-2,4-dimethyl-8-[2'-(1H-tetrazol-5-yl)-bi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Compound was evaluated for the in vitro inhibition of specific binding of [125I]-A II to Angiotensin II receptor, type 1 from rat adrenal membranes. | J Med Chem 41: 4251-60 (1998) Article DOI: 10.1021/jm970690q BindingDB Entry DOI: 10.7270/Q2FB55NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50469615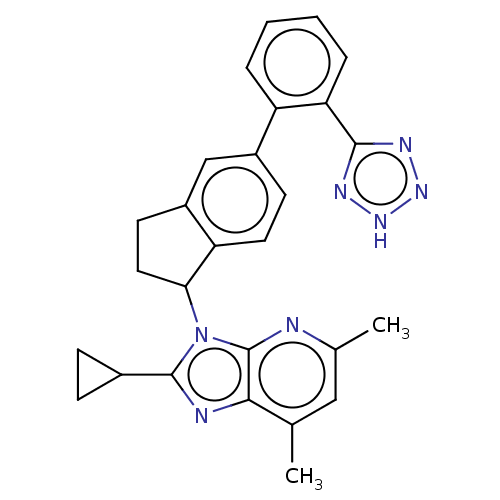 (CHEMBL174546) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity against Angiotensin II receptor, type 1 using [125I]Sar-Ile8-AII in rat liver membranes | Citation and Details Article DOI: 10.1016/S0960-894X(01)81128-8 BindingDB Entry DOI: 10.7270/Q2TB19MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50213781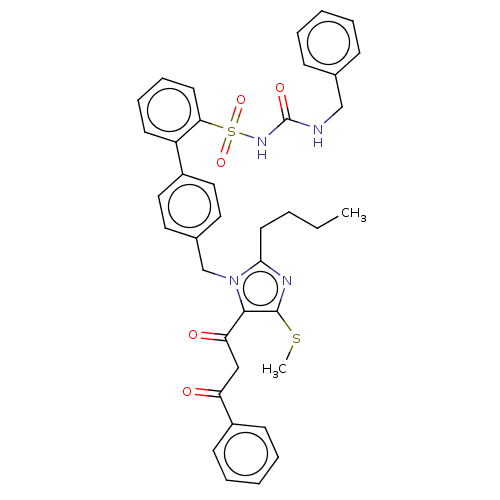 (CHEMBL327927) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Concentration required for 50% inhibition of [125I]- AII binding to rat liver membrane preparation (AT1) | Citation and Details BindingDB Entry DOI: 10.7270/Q2NC63CR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50472361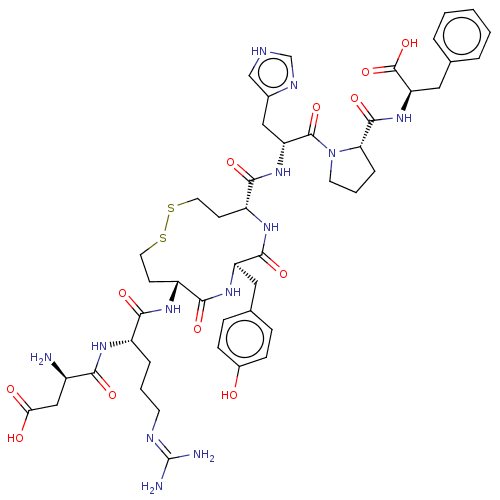 (CHEMBL406349) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description In vitro inhibitory activity against rat Angiotensin II receptor, type 1 expressed in CHO cells | J Med Chem 42: 4524-37 (1999) Article DOI: 10.1021/jm991089q BindingDB Entry DOI: 10.7270/Q2PN98C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470206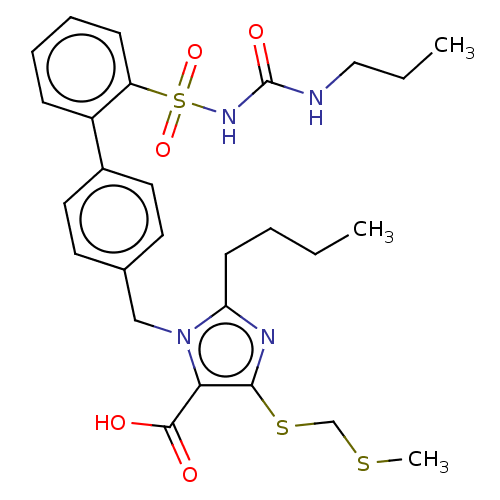 (CHEMBL445365) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50213569 (CHEMBL78866) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470225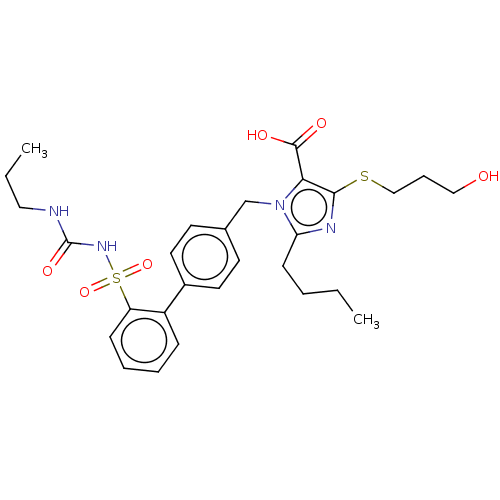 (CHEMBL80700) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470226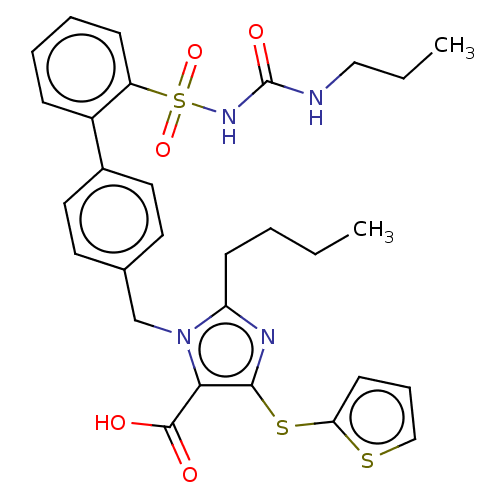 (CHEMBL311827) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470235 (CHEMBL308135) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50031491 (2-propyl-4-(methythio)-1-[[[2'-[(propylamino)carbo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50213557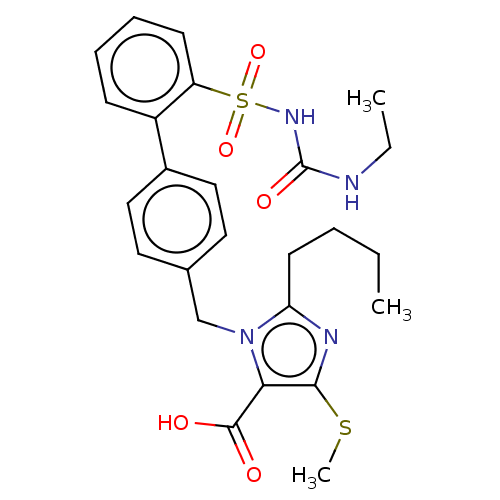 (CHEMBL309602) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50470250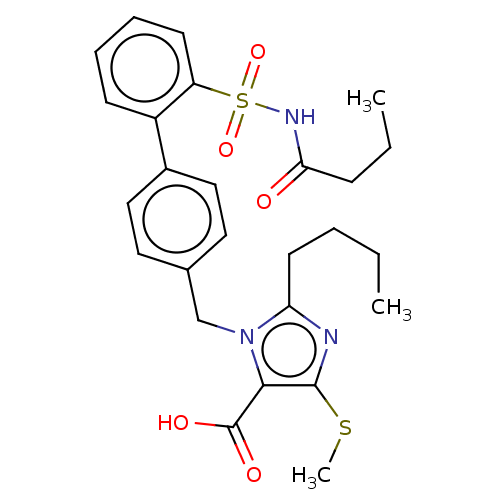 (CHEMBL77308) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Roussel PGU Cardiovascular Agents Curated by ChEMBL | Assay Description Specific binding inhibition of [125I]AII to Angiotensin II receptor, type 1 in rat liver membrane | J Med Chem 38: 2357-77 (1995) Article DOI: 10.1021/jm00013a013 BindingDB Entry DOI: 10.7270/Q2KK9FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50213550 (CHEMBL87847) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description In vitro displacement of [125I]angiotensin II from rat liver (Angiotensin 1 receptor) membrane preparation | Citation and Details BindingDB Entry DOI: 10.7270/Q2S184PR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 697 total ) | Next | Last >> |