

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50145722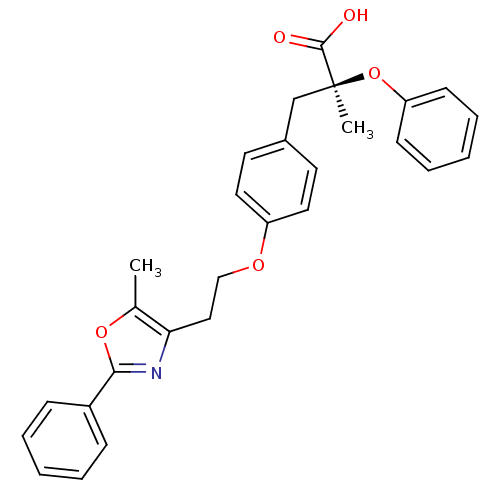 ((S)-2-Methyl-3-{4-[2-(5-methyl-2-phenyl-oxazol-4-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
TBA | Assay Description Transactivation of GAL4-fused Homo sapiens (human) PPARgamma DNA binding domain expressed in African green monkey CV1 cells by luciferase reporter ge... | Citation and Details Article DOI: 10.1007/s00044-012-0003-4 BindingDB Entry DOI: 10.7270/Q280544F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50145722 ((S)-2-Methyl-3-{4-[2-(5-methyl-2-phenyl-oxazol-4-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Transcriptional activation of reporter assay by PPAR gamma receptor in CV-1 cells | J Med Chem 47: 2422-5 (2004) Article DOI: 10.1021/jm0342616 BindingDB Entry DOI: 10.7270/Q2TQ6100 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50145722 ((S)-2-Methyl-3-{4-[2-(5-methyl-2-phenyl-oxazol-4-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
TBA | Assay Description Transactivation of Homo sapiens (human) PPARgamma assessed as luciferase activity by reporter gene assay | Citation and Details Article DOI: 10.1007/s00044-011-9818-7 BindingDB Entry DOI: 10.7270/Q2R49TNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50145722 ((S)-2-Methyl-3-{4-[2-(5-methyl-2-phenyl-oxazol-4-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
TBA | Assay Description Transactivation of GAL4-fused Homo sapiens (human) PPARgamma DNA binding domain expressed in African green monkey CV1 cells by luciferase reporter ge... | Citation and Details Article DOI: 10.1007/s00044-012-0003-4 BindingDB Entry DOI: 10.7270/Q280544F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50145722 ((S)-2-Methyl-3-{4-[2-(5-methyl-2-phenyl-oxazol-4-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
TBA | Assay Description Transactivation of Homo sapiens (human) PPARalpha assessed as luciferase activity by reporter gene assay | Citation and Details Article DOI: 10.1007/s00044-011-9818-7 BindingDB Entry DOI: 10.7270/Q2R49TNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50145722 ((S)-2-Methyl-3-{4-[2-(5-methyl-2-phenyl-oxazol-4-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Transcriptional activation of reporter assay by human Peroxisome proliferator activated receptor alpha in CV-1 cells | J Med Chem 47: 2422-5 (2004) Article DOI: 10.1021/jm0342616 BindingDB Entry DOI: 10.7270/Q2TQ6100 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50145722 ((S)-2-Methyl-3-{4-[2-(5-methyl-2-phenyl-oxazol-4-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Transactivation activity for human Peroxisome proliferator activated receptor alpha | Bioorg Med Chem Lett 14: 6113-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.031 BindingDB Entry DOI: 10.7270/Q2QN67J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50145722 ((S)-2-Methyl-3-{4-[2-(5-methyl-2-phenyl-oxazol-4-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
TBA | Assay Description Transactivation of GAL4-fused Homo sapiens (human) PPARalpha DNA binding domain expressed in African green monkey CV1 cells by luciferase reporter ge... | Citation and Details Article DOI: 10.1007/s00044-012-0003-4 BindingDB Entry DOI: 10.7270/Q280544F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50145722 ((S)-2-Methyl-3-{4-[2-(5-methyl-2-phenyl-oxazol-4-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
TBA | Assay Description Transactivation of GAL4-fused Homo sapiens (human) PPARalpha DNA binding domain expressed in African green monkey CV1 cells by luciferase reporter ge... | Citation and Details Article DOI: 10.1007/s00044-012-0003-4 BindingDB Entry DOI: 10.7270/Q280544F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Mus musculus) | BDBM50145722 ((S)-2-Methyl-3-{4-[2-(5-methyl-2-phenyl-oxazol-4-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Transactivation activity for mouse Peroxisome proliferator activated receptor alpha | Bioorg Med Chem Lett 14: 6113-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.031 BindingDB Entry DOI: 10.7270/Q2QN67J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||