

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236963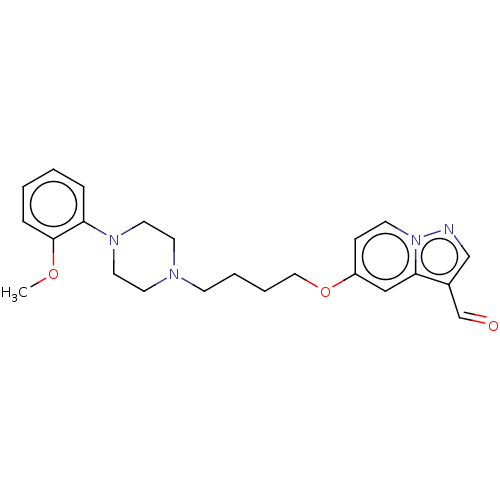 (CHEMBL4098236) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Concentration required for the inhibitory activity against human Farnesyltransferase | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50236963 (CHEMBL4098236) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Concentration required for the inhibitory activity against human Farnesyltransferase | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50236963 (CHEMBL4098236) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Agonist activity at human Dopamine D2S receptor expressed in HEK293T cell membranes coexpressing Galphai2 incubated for 30 mins measured after 75 min... | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50236963 (CHEMBL4098236) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a |
Friedrich-Alexander University Erlangen-N£rnberg Curated by ChEMBL | Assay Description Agonist activity at human Dopamine D2S receptor expressed in HEK293T cell membranes coexpressing GalphaoA incubated for 30 mins measured after 75 min... | J Med Chem 60: 2908-2929 (2017) Article DOI: 10.1021/acs.jmedchem.6b01857 BindingDB Entry DOI: 10.7270/Q2CJ8GR7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||