 Found 3 hits of ec50 for monomerid = 85050
Found 3 hits of ec50 for monomerid = 85050 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neurotensin receptor type 2
(Rattus norvegicus) | BDBM85050
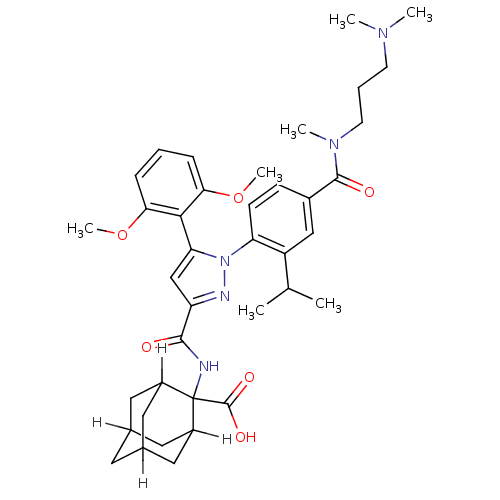
(CAS_184162-64-9 | SR 142948A | SR142948 | SR142948...)Show SMILES [H]C12CC3([H])CC([H])(C1)C(NC(=O)c1cc(-c4c(OC)cccc4OC)n(n1)-c1ccc(cc1C(C)C)C(=O)N(C)CCCN(C)C)(C(O)=O)C([H])(C2)C3 |TLB:8:6:53:1.52.2,8:1:6.9.5:53,10:9:3.5.53:1.8.52,THB:47:9:3.5.53:1.8.52,47:9:53:1.52.2,2:1:9:3.5.53,10:9:53:1.52.2,(4.26,2.59,;4.59,4.1,;3.08,4.03,;4.37,4.77,;5.38,3.61,;5.66,4.3,;6.94,4.77,;8.42,4.31,;5.99,3.36,;6.94,6.26,;8.48,6.12,;9.13,4.73,;8.25,3.46,;10.66,4.59,;11.67,5.75,;13.09,5.15,;14.41,5.94,;15.76,5.2,;16.31,3.76,;17.83,3.52,;17.08,5.99,;17.05,7.53,;15.7,8.28,;14.38,7.48,;13.04,8.23,;13.01,9.77,;12.96,3.62,;11.46,3.27,;13.7,2.27,;15.24,2.24,;15.99,.9,;15.2,-.42,;13.66,-.4,;12.91,.95,;11.37,.98,;10.58,-.34,;10.62,2.32,;15.94,-1.77,;17.48,-1.8,;15.15,-3.09,;13.61,-3.06,;15.9,-4.44,;15.1,-5.76,;15.85,-7.1,;15.06,-8.42,;15.8,-9.77,;13.52,-8.4,;7.34,7.74,;6.25,8.83,;8.6,8.63,;5.66,7,;5.66,8.54,;4.59,5.71,;4.37,6.26,)| Show InChI InChI=1S/C39H51N5O6/c1-23(2)29-21-26(37(46)43(5)15-9-14-42(3)4)12-13-31(29)44-32(35-33(49-6)10-8-11-34(35)50-7)22-30(41-44)36(45)40-39(38(47)48)27-17-24-16-25(19-27)20-28(39)18-24/h8,10-13,21-25,27-28H,9,14-20H2,1-7H3,(H,40,45)(H,47,48) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 18 | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Agonist activity at rat neurotensin receptor type 2 expressed in CHOK1 cells assessed as increase in calcium release by FLIPR assay |
Bioorg Med Chem Lett 25: 292-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.11.047
BindingDB Entry DOI: 10.7270/Q2M90B9G |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 2
(Rattus norvegicus) | BDBM85050

(CAS_184162-64-9 | SR 142948A | SR142948 | SR142948...)Show SMILES [H]C12CC3([H])CC([H])(C1)C(NC(=O)c1cc(-c4c(OC)cccc4OC)n(n1)-c1ccc(cc1C(C)C)C(=O)N(C)CCCN(C)C)(C(O)=O)C([H])(C2)C3 |TLB:8:6:53:1.52.2,8:1:6.9.5:53,10:9:3.5.53:1.8.52,THB:47:9:3.5.53:1.8.52,47:9:53:1.52.2,2:1:9:3.5.53,10:9:53:1.52.2,(4.26,2.59,;4.59,4.1,;3.08,4.03,;4.37,4.77,;5.38,3.61,;5.66,4.3,;6.94,4.77,;8.42,4.31,;5.99,3.36,;6.94,6.26,;8.48,6.12,;9.13,4.73,;8.25,3.46,;10.66,4.59,;11.67,5.75,;13.09,5.15,;14.41,5.94,;15.76,5.2,;16.31,3.76,;17.83,3.52,;17.08,5.99,;17.05,7.53,;15.7,8.28,;14.38,7.48,;13.04,8.23,;13.01,9.77,;12.96,3.62,;11.46,3.27,;13.7,2.27,;15.24,2.24,;15.99,.9,;15.2,-.42,;13.66,-.4,;12.91,.95,;11.37,.98,;10.58,-.34,;10.62,2.32,;15.94,-1.77,;17.48,-1.8,;15.15,-3.09,;13.61,-3.06,;15.9,-4.44,;15.1,-5.76,;15.85,-7.1,;15.06,-8.42,;15.8,-9.77,;13.52,-8.4,;7.34,7.74,;6.25,8.83,;8.6,8.63,;5.66,7,;5.66,8.54,;4.59,5.71,;4.37,6.26,)| Show InChI InChI=1S/C39H51N5O6/c1-23(2)29-21-26(37(46)43(5)15-9-14-42(3)4)12-13-31(29)44-32(35-33(49-6)10-8-11-34(35)50-7)22-30(41-44)36(45)40-39(38(47)48)27-17-24-16-25(19-27)20-28(39)18-24/h8,10-13,21-25,27-28H,9,14-20H2,1-7H3,(H,40,45)(H,47,48) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Agonist activity at rat NTS2 stably expressed in CHOK1 cells assessed as induction of calcium release by FLIPR assay |
J Med Chem 57: 5318-32 (2014)
Article DOI: 10.1021/jm5003843
BindingDB Entry DOI: 10.7270/Q2PR7XJD |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 2
(Rattus norvegicus) | BDBM85050

(CAS_184162-64-9 | SR 142948A | SR142948 | SR142948...)Show SMILES [H]C12CC3([H])CC([H])(C1)C(NC(=O)c1cc(-c4c(OC)cccc4OC)n(n1)-c1ccc(cc1C(C)C)C(=O)N(C)CCCN(C)C)(C(O)=O)C([H])(C2)C3 |TLB:8:6:53:1.52.2,8:1:6.9.5:53,10:9:3.5.53:1.8.52,THB:47:9:3.5.53:1.8.52,47:9:53:1.52.2,2:1:9:3.5.53,10:9:53:1.52.2,(4.26,2.59,;4.59,4.1,;3.08,4.03,;4.37,4.77,;5.38,3.61,;5.66,4.3,;6.94,4.77,;8.42,4.31,;5.99,3.36,;6.94,6.26,;8.48,6.12,;9.13,4.73,;8.25,3.46,;10.66,4.59,;11.67,5.75,;13.09,5.15,;14.41,5.94,;15.76,5.2,;16.31,3.76,;17.83,3.52,;17.08,5.99,;17.05,7.53,;15.7,8.28,;14.38,7.48,;13.04,8.23,;13.01,9.77,;12.96,3.62,;11.46,3.27,;13.7,2.27,;15.24,2.24,;15.99,.9,;15.2,-.42,;13.66,-.4,;12.91,.95,;11.37,.98,;10.58,-.34,;10.62,2.32,;15.94,-1.77,;17.48,-1.8,;15.15,-3.09,;13.61,-3.06,;15.9,-4.44,;15.1,-5.76,;15.85,-7.1,;15.06,-8.42,;15.8,-9.77,;13.52,-8.4,;7.34,7.74,;6.25,8.83,;8.6,8.63,;5.66,7,;5.66,8.54,;4.59,5.71,;4.37,6.26,)| Show InChI InChI=1S/C39H51N5O6/c1-23(2)29-21-26(37(46)43(5)15-9-14-42(3)4)12-13-31(29)44-32(35-33(49-6)10-8-11-34(35)50-7)22-30(41-44)36(45)40-39(38(47)48)27-17-24-16-25(19-27)20-28(39)18-24/h8,10-13,21-25,27-28H,9,14-20H2,1-7H3,(H,40,45)(H,47,48) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 22 | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Agonist activity at rat NTS2 receptor expressed in CHO-K1 cells assessed as calcium mobilization by FLIPR assay |
J Med Chem 57: 7472-7 (2014)
Article DOI: 10.1021/jm500857r
BindingDB Entry DOI: 10.7270/Q2BP04C6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data


