

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bifunctional dihydrofolate reductase-thymidylate synthase (Cryptosporidium hominis) | BDBM25826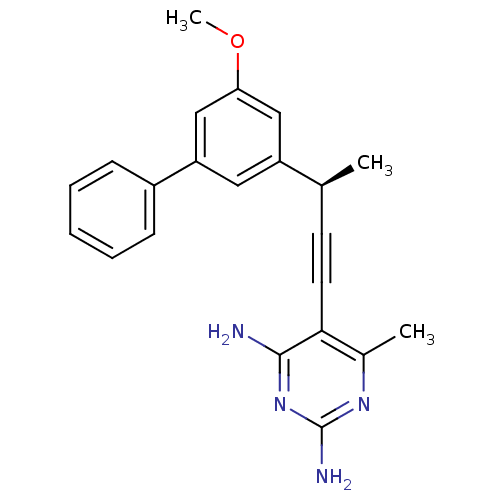 (5-[(3R)-3-(3-methoxy-5-phenylphenyl)but-1-yn-1-yl]...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.0 | 25 |
University of Connecticut at Storrs | Assay Description Enzyme activity assays were performed by monitoring the change in UV absorbance at 340 nm. Enzyme assays were performed at least four times. IC50 val... | J Med Chem 51: 6839-52 (2008) Article DOI: 10.1021/jm8009124 BindingDB Entry DOI: 10.7270/Q2W37TMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Cryptosporidium hominis) | BDBM25826 (5-[(3R)-3-(3-methoxy-5-phenylphenyl)but-1-yn-1-yl]...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Compounds were evaluated in spectrophotometric enzyme assays using ChDHFR-TS and hDHFR. Inhibition constants (IC50) were measured (see Table 4). The ... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Candida albicans) | BDBM25826 (5-[(3R)-3-(3-methoxy-5-phenylphenyl)but-1-yn-1-yl]...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of Candida albicans DHFR expressed in Escherichia coli BL21 (DE3) assessed as rate of NADPH consumption using dihydrofolate as susbtrate | Bioorg Med Chem 17: 4866-72 (2009) Article DOI: 10.1016/j.bmc.2009.06.021 BindingDB Entry DOI: 10.7270/Q2JM29P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Staphylococcus aureus) | BDBM25826 (5-[(3R)-3-(3-methoxy-5-phenylphenyl)but-1-yn-1-yl]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Oklahoma State University Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus wild type recombinant DHFR by MTS assay | Antimicrob Agents Chemother 54: 3825-33 (2010) Article DOI: 10.1128/AAC.00361-10 BindingDB Entry DOI: 10.7270/Q25M6604 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM25826 (5-[(3R)-3-(3-methoxy-5-phenylphenyl)but-1-yn-1-yl]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut Curated by ChEMBL | Assay Description Inhibition of human DHFR assessed as rate of NADPH consumption using dihydrofolate as susbtrate | Bioorg Med Chem 17: 4866-72 (2009) Article DOI: 10.1016/j.bmc.2009.06.021 BindingDB Entry DOI: 10.7270/Q2JM29P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM25826 (5-[(3R)-3-(3-methoxy-5-phenylphenyl)but-1-yn-1-yl]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.36E+3 | n/a | n/a | n/a | n/a | 7.0 | 25 |
University of Connecticut at Storrs | Assay Description Enzyme activity assays were performed by monitoring the change in UV absorbance at 340 nm. Enzyme assays were performed at least four times. IC50 val... | J Med Chem 51: 6839-52 (2008) Article DOI: 10.1021/jm8009124 BindingDB Entry DOI: 10.7270/Q2W37TMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM25826 (5-[(3R)-3-(3-methoxy-5-phenylphenyl)but-1-yn-1-yl]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 1.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut US Patent | Assay Description Compounds were evaluated in spectrophotometric enzyme assays using ChDHFR-TS and hDHFR. Inhibition constants (IC50) were measured (see Table 4). The ... | US Patent US8853228 (2014) BindingDB Entry DOI: 10.7270/Q2TD9W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||