

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thromboxane-A synthase (Homo sapiens (Human)) | BDBM50000317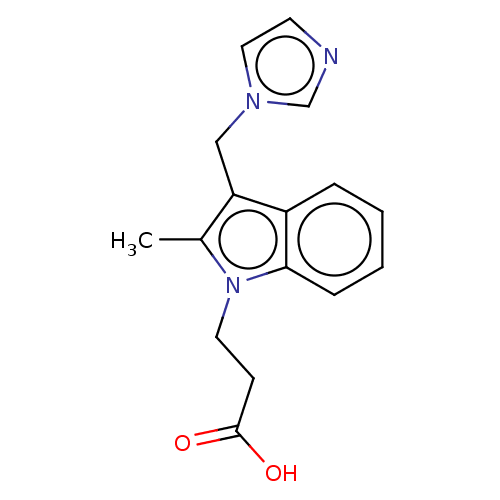 (3-(3-Imidazol-1-ylmethyl-2-methyl-indol-1-yl)-prop...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against human platelet microsomal TXA2 synthase | Bioorg Med Chem Lett 6: 1691-1696 (1996) Article DOI: 10.1016/0960-894X(96)00299-5 BindingDB Entry DOI: 10.7270/Q27P8ZBS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (Homo sapiens (Human)) | BDBM50000317 (3-(3-Imidazol-1-ylmethyl-2-methyl-indol-1-yl)-prop...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description Activity against human platelet microsome Thromboxane synthase | J Med Chem 40: 3442-52 (1997) Article DOI: 10.1021/jm9702793 BindingDB Entry DOI: 10.7270/Q2Z0378X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (Homo sapiens (Human)) | BDBM50000317 (3-(3-Imidazol-1-ylmethyl-2-methyl-indol-1-yl)-prop...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of TXA2 synthetase from human platelets | J Med Chem 29: 342-6 (1986) BindingDB Entry DOI: 10.7270/Q2W66JSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (Ovis aries (Sheep)) | BDBM50000317 (3-(3-Imidazol-1-ylmethyl-2-methyl-indol-1-yl)-prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Prostaglandin G/H synthase from ram seminal vesicle microsomes | J Med Chem 29: 342-6 (1986) BindingDB Entry DOI: 10.7270/Q2W66JSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin synthase (Homo sapiens (Human)) | BDBM50000317 (3-(3-Imidazol-1-ylmethyl-2-methyl-indol-1-yl)-prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of PGI-2 synthetase from porcine aorta | J Med Chem 29: 342-6 (1986) BindingDB Entry DOI: 10.7270/Q2W66JSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Rattus norvegicus) | BDBM50000317 (3-(3-Imidazol-1-ylmethyl-2-methyl-indol-1-yl)-prop...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of 11 beta-hydroxylase from rat adrenal gland | J Med Chem 29: 342-6 (1986) BindingDB Entry DOI: 10.7270/Q2W66JSZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||