

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A disintegrin and metalloproteinase with thrombospondin motifs 5 (Homo sapiens (Human)) | BDBM50033807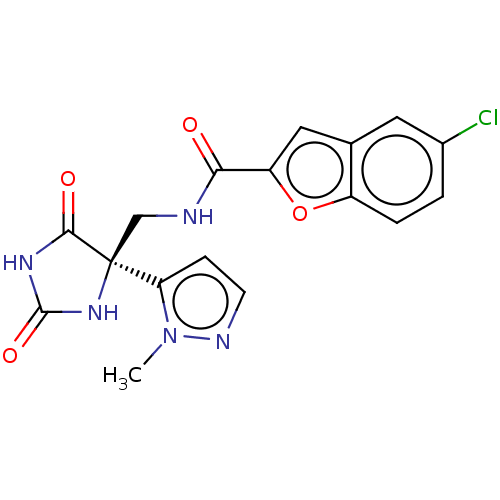 (CHEMBL3358157) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human ADAMTS-5 using VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG peptide substrate by AlphaScreen assay | J Med Chem 57: 10476-85 (2014) Article DOI: 10.1021/jm501522n BindingDB Entry DOI: 10.7270/Q2K35W85 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| A disintegrin and metalloproteinase with thrombospondin motifs 4 (Homo sapiens (Human)) | BDBM50033807 (CHEMBL3358157) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human ADAMTS-4 using VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG peptide substrate by AlphaScreen assay | J Med Chem 57: 10476-85 (2014) Article DOI: 10.1021/jm501522n BindingDB Entry DOI: 10.7270/Q2K35W85 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| A disintegrin and metalloproteinase with thrombospondin motifs 5 (Homo sapiens (Human)) | BDBM50033807 (CHEMBL3358157) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human ADAMTS-5 using VQTVTWPDMELPLPRNITEGEARGSVILTVKPIFEVSPSPLKG peptide substrate by AlphaScreen assay in presence of 50% rat plasma | J Med Chem 57: 10476-85 (2014) Article DOI: 10.1021/jm501522n BindingDB Entry DOI: 10.7270/Q2K35W85 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50033807 (CHEMBL3358157) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human MMP13 using Mca-PQG1 peptide substrate assessed as substrate cleavage after 2 to 4 hrs | J Med Chem 57: 10476-85 (2014) Article DOI: 10.1021/jm501522n BindingDB Entry DOI: 10.7270/Q2K35W85 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50033807 (CHEMBL3358157) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human MMP2 using Mca-PQG1 peptide substrate assessed as substrate cleavage after 2 to 4 hrs | J Med Chem 57: 10476-85 (2014) Article DOI: 10.1021/jm501522n BindingDB Entry DOI: 10.7270/Q2K35W85 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-14 (Homo sapiens (Human)) | BDBM50033807 (CHEMBL3358157) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human MMP14 using Mca-PQG1 peptide substrate assessed as substrate cleavage after 2 to 4 hrs | J Med Chem 57: 10476-85 (2014) Article DOI: 10.1021/jm501522n BindingDB Entry DOI: 10.7270/Q2K35W85 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50033807 (CHEMBL3358157) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Inhibition of human MMP3 using Mca-PQG1 peptide substrate assessed as substrate cleavage after 2 to 4 hrs | J Med Chem 57: 10476-85 (2014) Article DOI: 10.1021/jm501522n BindingDB Entry DOI: 10.7270/Q2K35W85 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||