 Found 10 hits of ic50 for monomerid = 50056315
Found 10 hits of ic50 for monomerid = 50056315 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Genome polyprotein
(Dengue virus) | BDBM50056315
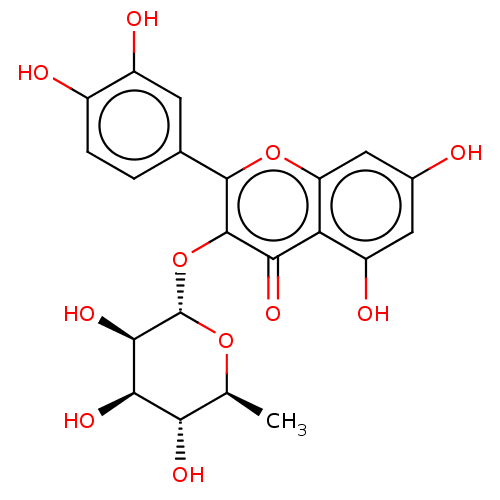
(CHEBI:17558 | Quercitrin)Show SMILES C[C@@H]1O[C@@H](Oc2c(oc3cc(O)cc(O)c3c2=O)-c2ccc(O)c(O)c2)[C@H](O)[C@H](O)[C@H]1O Show InChI InChI=1S/C21H20O11/c1-7-15(26)17(28)18(29)21(30-7)32-20-16(27)14-12(25)5-9(22)6-13(14)31-19(20)8-2-3-10(23)11(24)4-8/h2-7,15,17-18,21-26,28-29H,1H3/t7-,15-,17+,18+,21-/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Queensland University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of dengue virus NS5 RNA dependent RNA polymerase |
Eur J Med Chem 176: 431-455 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.010
BindingDB Entry DOI: 10.7270/Q20R9STW |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50056315

(CHEBI:17558 | Quercitrin)Show SMILES C[C@@H]1O[C@@H](Oc2c(oc3cc(O)cc(O)c3c2=O)-c2ccc(O)c(O)c2)[C@H](O)[C@H](O)[C@H]1O Show InChI InChI=1S/C21H20O11/c1-7-15(26)17(28)18(29)21(30-7)32-20-16(27)14-12(25)5-9(22)6-13(14)31-19(20)8-2-3-10(23)11(24)4-8/h2-7,15,17-18,21-26,28-29H,1H3/t7-,15-,17+,18+,21-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of sEH (unknown origin) assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate measured after 40 mins by f... |
Citation and Details
Article DOI: 10.1016/j.bmc.2016.05.034
BindingDB Entry DOI: 10.7270/Q2833WP0 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50056315

(CHEBI:17558 | Quercitrin)Show SMILES C[C@@H]1O[C@@H](Oc2c(oc3cc(O)cc(O)c3c2=O)-c2ccc(O)c(O)c2)[C@H](O)[C@H](O)[C@H]1O Show InChI InChI=1S/C21H20O11/c1-7-15(26)17(28)18(29)21(30-7)32-20-16(27)14-12(25)5-9(22)6-13(14)31-19(20)8-2-3-10(23)11(24)4-8/h2-7,15,17-18,21-26,28-29H,1H3/t7-,15-,17+,18+,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50056315

(CHEBI:17558 | Quercitrin)Show SMILES C[C@@H]1O[C@@H](Oc2c(oc3cc(O)cc(O)c3c2=O)-c2ccc(O)c(O)c2)[C@H](O)[C@H](O)[C@H]1O Show InChI InChI=1S/C21H20O11/c1-7-15(26)17(28)18(29)21(30-7)32-20-16(27)14-12(25)5-9(22)6-13(14)31-19(20)8-2-3-10(23)11(24)4-8/h2-7,15,17-18,21-26,28-29H,1H3/t7-,15-,17+,18+,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of PTP1B (unknown origin) using pNPP substrate measured after 3 mins by colorimetric assay |
Bioorg Med Chem Lett 25: 2028-32 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.003
BindingDB Entry DOI: 10.7270/Q2XP76M3 |
More data for this
Ligand-Target Pair | |
Solute carrier organic anion transporter family member 2B1
(Homo sapiens) | BDBM50056315

(CHEBI:17558 | Quercitrin)Show SMILES C[C@@H]1O[C@@H](Oc2c(oc3cc(O)cc(O)c3c2=O)-c2ccc(O)c(O)c2)[C@H](O)[C@H](O)[C@H]1O Show InChI InChI=1S/C21H20O11/c1-7-15(26)17(28)18(29)21(30-7)32-20-16(27)14-12(25)5-9(22)6-13(14)31-19(20)8-2-3-10(23)11(24)4-8/h2-7,15,17-18,21-26,28-29H,1H3/t7-,15-,17+,18+,21-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 6.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50056315

(CHEBI:17558 | Quercitrin)Show SMILES C[C@@H]1O[C@@H](Oc2c(oc3cc(O)cc(O)c3c2=O)-c2ccc(O)c(O)c2)[C@H](O)[C@H](O)[C@H]1O Show InChI InChI=1S/C21H20O11/c1-7-15(26)17(28)18(29)21(30-7)32-20-16(27)14-12(25)5-9(22)6-13(14)31-19(20)8-2-3-10(23)11(24)4-8/h2-7,15,17-18,21-26,28-29H,1H3/t7-,15-,17+,18+,21-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pretoria
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase |
J Nat Prod 81: 49-56 (2018)
Article DOI: 10.1021/acs.jnatprod.7b00564
BindingDB Entry DOI: 10.7270/Q2H70JBS |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus (strain A/USSR/90/1977 H1N1)) | BDBM50056315

(CHEBI:17558 | Quercitrin)Show SMILES C[C@@H]1O[C@@H](Oc2c(oc3cc(O)cc(O)c3c2=O)-c2ccc(O)c(O)c2)[C@H](O)[C@H](O)[C@H]1O Show InChI InChI=1S/C21H20O11/c1-7-15(26)17(28)18(29)21(30-7)32-20-16(27)14-12(25)5-9(22)6-13(14)31-19(20)8-2-3-10(23)11(24)4-8/h2-7,15,17-18,21-26,28-29H,1H3/t7-,15-,17+,18+,21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.09E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universitaet Berlin
Curated by ChEMBL
| Assay Description
Inhibition of influenza A virus (A/California/07/2009(H1N1)) pdm09 neuraminidase using MUNANA substrate pre-incubated for 30 mins before substrate ad... |
Bioorg Med Chem Lett 24: 4312-7 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.010
BindingDB Entry DOI: 10.7270/Q2BC4164 |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus (strain A/USSR/90/1977 H1N1)) | BDBM50056315

(CHEBI:17558 | Quercitrin)Show SMILES C[C@@H]1O[C@@H](Oc2c(oc3cc(O)cc(O)c3c2=O)-c2ccc(O)c(O)c2)[C@H](O)[C@H](O)[C@H]1O Show InChI InChI=1S/C21H20O11/c1-7-15(26)17(28)18(29)21(30-7)32-20-16(27)14-12(25)5-9(22)6-13(14)31-19(20)8-2-3-10(23)11(24)4-8/h2-7,15,17-18,21-26,28-29H,1H3/t7-,15-,17+,18+,21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.21E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Freie Universitaet Berlin
Curated by ChEMBL
| Assay Description
Inhibition of influenza A virus (A/Perth/16/2009(H3N2)) neuraminidase using MUNANA substrate pre-incubated for 30 mins before substrate addition by f... |
Bioorg Med Chem Lett 24: 4312-7 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.010
BindingDB Entry DOI: 10.7270/Q2BC4164 |
More data for this
Ligand-Target Pair | |
Polyphenol oxidase 2
(Agaricus bisporus (Common mushroom)) | BDBM50056315

(CHEBI:17558 | Quercitrin)Show SMILES C[C@@H]1O[C@@H](Oc2c(oc3cc(O)cc(O)c3c2=O)-c2ccc(O)c(O)c2)[C@H](O)[C@H](O)[C@H]1O Show InChI InChI=1S/C21H20O11/c1-7-15(26)17(28)18(29)21(30-7)32-20-16(27)14-12(25)5-9(22)6-13(14)31-19(20)8-2-3-10(23)11(24)4-8/h2-7,15,17-18,21-26,28-29H,1H3/t7-,15-,17+,18+,21-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pretoria
Curated by ChEMBL
| Assay Description
Inhibition of mushroom tyrosinase using L-tyrosine as substrate pretreated for 5 mins followed by substrate addition measured over 30 mins by spectro... |
J Nat Prod 81: 49-56 (2018)
Article DOI: 10.1021/acs.jnatprod.7b00564
BindingDB Entry DOI: 10.7270/Q2H70JBS |
More data for this
Ligand-Target Pair | |
Integrase
(Human immunodeficiency virus 1) | BDBM50056315

(CHEBI:17558 | Quercitrin)Show SMILES C[C@@H]1O[C@@H](Oc2c(oc3cc(O)cc(O)c3c2=O)-c2ccc(O)c(O)c2)[C@H](O)[C@H](O)[C@H]1O Show InChI InChI=1S/C21H20O11/c1-7-15(26)17(28)18(29)21(30-7)32-20-16(27)14-12(25)5-9(22)6-13(14)31-19(20)8-2-3-10(23)11(24)4-8/h2-7,15,17-18,21-26,28-29H,1H3/t7-,15-,17+,18+,21-/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.67E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science & Technology
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 recombinant integrase expressed in Escherichia coli |
J Nat Prod 61: 145-8 (1998)
Article DOI: 10.1021/np970171q
BindingDB Entry DOI: 10.7270/Q2V127NW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data