

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50096122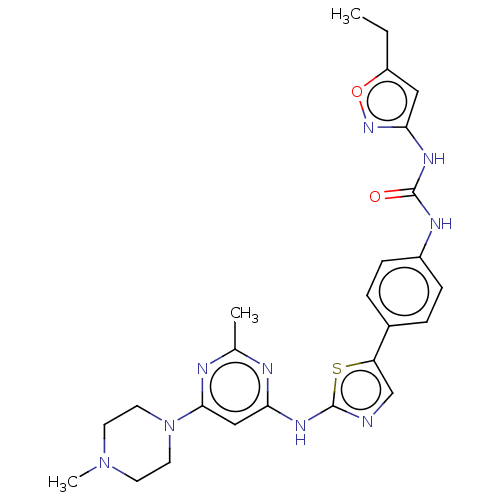 (CHEMBL3593288) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of recombinant GST-tagged VEGFR2 kinase domain (V789 to V1356) (unknown origin) expressed in insect Sf9 cells using polyGlu4:Tyr peptide a... | Eur J Med Chem 100: 151-61 (2015) Article DOI: 10.1016/j.ejmech.2015.05.008 BindingDB Entry DOI: 10.7270/Q25H7J12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50096122 (CHEMBL3593288) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of recombinant GST-tagged Aurora-A kinase domain (S123 to S401) (unknown origin) expressed in insect Sf9 cells using tetra(LRRASLG) peptid... | Eur J Med Chem 100: 151-61 (2015) Article DOI: 10.1016/j.ejmech.2015.05.008 BindingDB Entry DOI: 10.7270/Q25H7J12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50096122 (CHEMBL3593288) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Inhibition of wild type GST-tagged FLT3 kinase domain (Y567 to S993) (unknown origin) expressed in baculovirus infected insect Sf9 cells using Her2 p... | Eur J Med Chem 100: 151-61 (2015) Article DOI: 10.1016/j.ejmech.2015.05.008 BindingDB Entry DOI: 10.7270/Q25H7J12 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||