

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50108052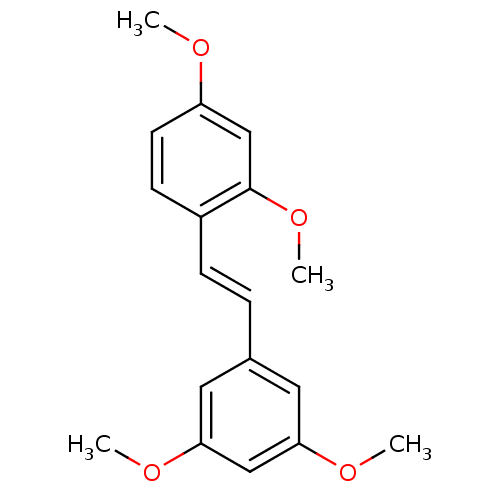 (1-[(E)-2-(3,5-dimethoxyphenyl)vinyl]-2,4-dimethoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Medicinal Chemistry, Endocrinology and Nephrology Unit, CHU de Québec - Research Center, Québec, Québec, Canada; Department of Molecular Medicine, Faculty of Medicine, Université Laval, Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP1B1 expressed in Escherichia coli DH5alpha using ethoxyresorufin as substrate preincubated for 3 mins followed by ... | Eur J Med Chem 135: 296-306 (2017) Article DOI: 10.1016/j.ejmech.2017.04.042 BindingDB Entry DOI: 10.7270/Q26Q20QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50108052 (1-[(E)-2-(3,5-dimethoxyphenyl)vinyl]-2,4-dimethoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50108052 (1-[(E)-2-(3,5-dimethoxyphenyl)vinyl]-2,4-dimethoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP1B1 expressed in Escherichia coli membranes co-expressing NADPH-P450 reductase assessed as reduction in ethoxyreso... | ACS Med Chem Lett 9: 1247-1252 (2018) Article DOI: 10.1021/acsmedchemlett.8b00409 BindingDB Entry DOI: 10.7270/Q2V1284K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50108052 (1-[(E)-2-(3,5-dimethoxyphenyl)vinyl]-2,4-dimethoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Whittier College Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP1B1 expressed in Escherichia coli DH5aplha cells assessed as O-deethylation of ethoxyresorufin in presence of NADP... | Bioorg Med Chem Lett 26: 3243-3247 (2016) Article DOI: 10.1016/j.bmcl.2016.05.064 BindingDB Entry DOI: 10.7270/Q2XG9T1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50108052 (1-[(E)-2-(3,5-dimethoxyphenyl)vinyl]-2,4-dimethoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of ethoxyresorufin O-deethylation (EROD) in bicistronic bacterial membranes expressing human cytochrome P450 1B1 | J Med Chem 45: 160-4 (2001) BindingDB Entry DOI: 10.7270/Q2668CGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50108052 (1-[(E)-2-(3,5-dimethoxyphenyl)vinyl]-2,4-dimethoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50108052 (1-[(E)-2-(3,5-dimethoxyphenyl)vinyl]-2,4-dimethoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | n/a | n/a | 209 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50108052 (1-[(E)-2-(3,5-dimethoxyphenyl)vinyl]-2,4-dimethoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of ethoxyresorufin O-deethylation (EROD) in bicistronic bacterial membranes epressing human cytochrome P450 1A1 | J Med Chem 45: 160-4 (2001) BindingDB Entry DOI: 10.7270/Q2668CGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50108052 (1-[(E)-2-(3,5-dimethoxyphenyl)vinyl]-2,4-dimethoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Medicinal Chemistry, Endocrinology and Nephrology Unit, CHU de Québec - Research Center, Québec, Québec, Canada; Department of Molecular Medicine, Faculty of Medicine, Université Laval, Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP1A1 expressed in Escherichia coli DH5alpha using ethoxyresorufin as substrate preincubated for 3 mins followed by ... | Eur J Med Chem 135: 296-306 (2017) Article DOI: 10.1016/j.ejmech.2017.04.042 BindingDB Entry DOI: 10.7270/Q26Q20QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50108052 (1-[(E)-2-(3,5-dimethoxyphenyl)vinyl]-2,4-dimethoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50108052 (1-[(E)-2-(3,5-dimethoxyphenyl)vinyl]-2,4-dimethoxy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP1A1 expressed in Escherichia coli membranes co-expressing NADPH-P450 reductase assessed as reduction in ethoxyreso... | ACS Med Chem Lett 9: 1247-1252 (2018) Article DOI: 10.1021/acsmedchemlett.8b00409 BindingDB Entry DOI: 10.7270/Q2V1284K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50108052 (1-[(E)-2-(3,5-dimethoxyphenyl)vinyl]-2,4-dimethoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50108052 (1-[(E)-2-(3,5-dimethoxyphenyl)vinyl]-2,4-dimethoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Medicinal Chemistry, Endocrinology and Nephrology Unit, CHU de Québec - Research Center, Québec, Québec, Canada; Department of Molecular Medicine, Faculty of Medicine, Université Laval, Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP1A2 expressed in Escherichia coli DH5alpha using ethoxyresorufin as substrate preincubated for 3 mins followed by ... | Eur J Med Chem 135: 296-306 (2017) Article DOI: 10.1016/j.ejmech.2017.04.042 BindingDB Entry DOI: 10.7270/Q26Q20QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50108052 (1-[(E)-2-(3,5-dimethoxyphenyl)vinyl]-2,4-dimethoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50108052 (1-[(E)-2-(3,5-dimethoxyphenyl)vinyl]-2,4-dimethoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP1A2 expressed in Escherichia coli membranes co-expressing NADPH-P450 reductase assessed as reduction in ethoxyreso... | ACS Med Chem Lett 9: 1247-1252 (2018) Article DOI: 10.1021/acsmedchemlett.8b00409 BindingDB Entry DOI: 10.7270/Q2V1284K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50108052 (1-[(E)-2-(3,5-dimethoxyphenyl)vinyl]-2,4-dimethoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition of ethoxyresorufin O-deethylation (EROD) in bicistronic bacterial membranes expressing human cytochrome P450 1A2 | J Med Chem 45: 160-4 (2001) BindingDB Entry DOI: 10.7270/Q2668CGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Homo sapiens (Human)) | BDBM50108052 (1-[(E)-2-(3,5-dimethoxyphenyl)vinyl]-2,4-dimethoxy...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chulalongkorn University Curated by ChEMBL | Assay Description Inhibition of tyrosinase | Bioorg Med Chem Lett 16: 5650-3 (2006) Article DOI: 10.1016/j.bmcl.2006.08.018 BindingDB Entry DOI: 10.7270/Q2348K00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||