 Found 2 hits of ic50 for monomerid = 50140090
Found 2 hits of ic50 for monomerid = 50140090 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
G2/mitotic-specific cyclin-B1
(Rattus norvegicus) | BDBM50140090
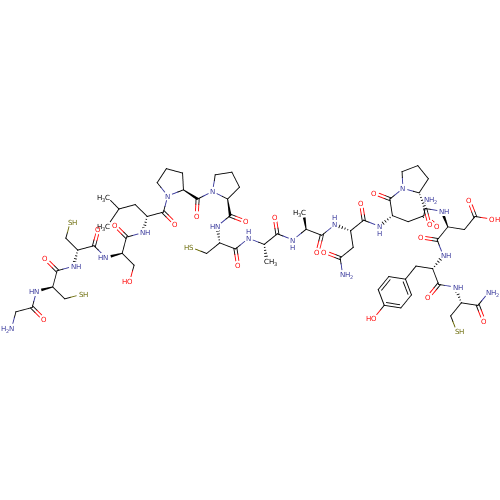
(CHEMBL217150 | RDPCCSNPVCTVHNPQIC*)Show SMILES CC(C)C[C@@H](NC(=O)[C@@H](CO)NC(=O)[C@@H](CS)NC(=O)[C@@H](CS)NC(=O)CN)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](CS)C(=O)N[C@@H](C)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N1CCC[C@@H]1C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CS)C(N)=O Show InChI InChI=1S/C65H99N19O22S4/c1-29(2)18-37(76-57(98)39(24-85)78-60(101)43(28-110)80-59(100)41(26-108)72-49(89)23-66)63(104)84-17-7-10-46(84)65(106)83-16-6-9-45(83)62(103)81-42(27-109)58(99)71-30(3)52(93)70-31(4)53(94)73-35(20-47(67)87)55(96)77-38(21-48(68)88)64(105)82-15-5-8-44(82)61(102)75-36(22-50(90)91)56(97)74-34(19-32-11-13-33(86)14-12-32)54(95)79-40(25-107)51(69)92/h11-14,29-31,34-46,85-86,107-110H,5-10,15-28,66H2,1-4H3,(H2,67,87)(H2,68,88)(H2,69,92)(H,70,93)(H,71,99)(H,72,89)(H,73,94)(H,74,97)(H,75,102)(H,76,98)(H,77,96)(H,78,101)(H,79,95)(H,80,100)(H,81,103)(H,90,91)/t30-,31-,34-,35-,36-,37+,38-,39+,40-,41+,42-,43+,44+,45-,46-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of rat Nicotinic acetylcholine receptor alpha7 (nAChR) in Xenopus Oocytes |
J Med Chem 47: 1234-41 (2004)
Article DOI: 10.1021/jm031010o
BindingDB Entry DOI: 10.7270/Q2RN378M |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3/beta-2
(Rattus norvegicus (Rat)) | BDBM50140090

(CHEMBL217150 | RDPCCSNPVCTVHNPQIC*)Show SMILES CC(C)C[C@@H](NC(=O)[C@@H](CO)NC(=O)[C@@H](CS)NC(=O)[C@@H](CS)NC(=O)CN)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)N[C@@H](CS)C(=O)N[C@@H](C)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N1CCC[C@@H]1C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CS)C(N)=O Show InChI InChI=1S/C65H99N19O22S4/c1-29(2)18-37(76-57(98)39(24-85)78-60(101)43(28-110)80-59(100)41(26-108)72-49(89)23-66)63(104)84-17-7-10-46(84)65(106)83-16-6-9-45(83)62(103)81-42(27-109)58(99)71-30(3)52(93)70-31(4)53(94)73-35(20-47(67)87)55(96)77-38(21-48(68)88)64(105)82-15-5-8-44(82)61(102)75-36(22-50(90)91)56(97)74-34(19-32-11-13-33(86)14-12-32)54(95)79-40(25-107)51(69)92/h11-14,29-31,34-46,85-86,107-110H,5-10,15-28,66H2,1-4H3,(H2,67,87)(H2,68,88)(H2,69,92)(H,70,93)(H,71,99)(H,72,89)(H,73,94)(H,74,97)(H,75,102)(H,76,98)(H,77,96)(H,78,101)(H,79,95)(H,80,100)(H,81,103)(H,90,91)/t30-,31-,34-,35-,36-,37+,38-,39+,40-,41+,42-,43+,44+,45-,46-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of rat Nicotinic acetylcholine receptor alpha3-beta2 expressed in Xenopus Oocytes |
J Med Chem 47: 1234-41 (2004)
Article DOI: 10.1021/jm031010o
BindingDB Entry DOI: 10.7270/Q2RN378M |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

