

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuronal acetylcholine receptor subunit alpha-4/alpha-5/beta-2 (Homo sapiens (Human)) | BDBM50241748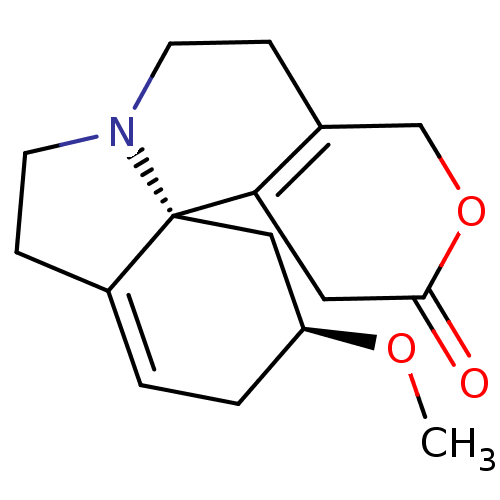 (CHEMBL518059 | dihydro-beta-erythroidine) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chile Curated by ChEMBL | Assay Description Antagonist activity at (alpha4beta2)2alpha5 nAChR (unknown origin) expressed in Xenopus oocytes assessed as inhibition of acetylcholine-induced curre... | Bioorg Med Chem 21: 2687-94 (2013) Article DOI: 10.1016/j.bmc.2013.03.024 BindingDB Entry DOI: 10.7270/Q2MW2JJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM50241748 (CHEMBL518059 | dihydro-beta-erythroidine) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chile Curated by ChEMBL | Assay Description Antagonist activity at (alpha4beta2)2alpha4 nAChR (unknown origin) expressed in Xenopus oocytes assessed as inhibition of acetylcholine-induced curre... | Bioorg Med Chem 21: 2687-94 (2013) Article DOI: 10.1016/j.bmc.2013.03.024 BindingDB Entry DOI: 10.7270/Q2MW2JJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM50241748 (CHEMBL518059 | dihydro-beta-erythroidine) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Chile Curated by ChEMBL | Assay Description Antagonist activity at (alpha4beta2)2beta2 nAChR (unknown origin) expressed in Xenopus oocytes assessed as inhibition of acetylcholine-induced curren... | Bioorg Med Chem 21: 2687-94 (2013) Article DOI: 10.1016/j.bmc.2013.03.024 BindingDB Entry DOI: 10.7270/Q2MW2JJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3 (Rattus norvegicus (Rat)) | BDBM50241748 (CHEMBL518059 | dihydro-beta-erythroidine) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Activity at rat alpha3beta4 nicotinic receptor expressed in KXalpha3beta4R2 cells assessed as nicotine-induced 86Rb+ efflux | J Nat Prod 69: 1514-6 (2006) Article DOI: 10.1021/np060291a BindingDB Entry DOI: 10.7270/Q2445M85 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||