

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intestinal-type alkaline phosphatase (Homo sapiens (Human)) | BDBM50242280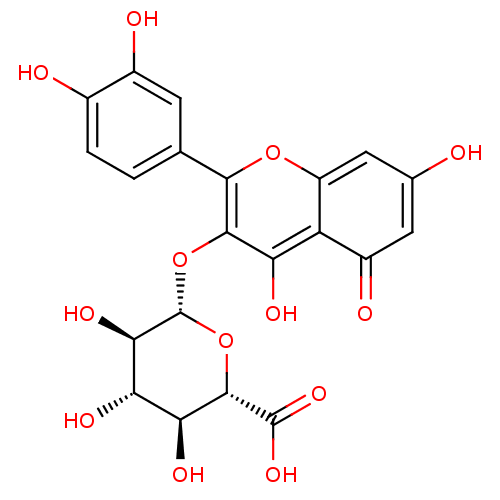 ((2S,3S,4S,5R,6S)-6-(2-(3,4-dihydroxyphenyl)-5,7-di...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | 891 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute(SBMRI, San Diego, CA... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2154FH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Intestinal-type alkaline phosphatase (Homo sapiens (Human)) | BDBM50242280 ((2S,3S,4S,5R,6S)-6-(2-(3,4-dihydroxyphenyl)-5,7-di...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | 3.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2RV0M5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Intestinal-type alkaline phosphatase 1 (Rattus norvegicus (Rat)) | BDBM50242280 ((2S,3S,4S,5R,6S)-6-(2-(3,4-dihydroxyphenyl)-5,7-di...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | 3.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q25Q4TJ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M17 leucyl aminopeptidase (Plasmodium falciparum 3D7) | BDBM50242280 ((2S,3S,4S,5R,6S)-6-(2-(3,4-dihydroxyphenyl)-5,7-di...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | 4.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SRMLSC Curated by PubChem BioAssay | Assay Description Southern Research Molecular Libraries Screening Center (SRMLSC) Southern Research Institute (Birmingham, Alabama) NIH Molecular Libraries Screening... | PubChem Bioassay (2009) BindingDB Entry DOI: 10.7270/Q27W69N8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, tissue-nonspecific isozyme (Homo sapiens (Human)) | BDBM50242280 ((2S,3S,4S,5R,6S)-6-(2-(3,4-dihydroxyphenyl)-5,7-di...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PCBioAssay | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute(SBMRI, San Diego, CA... | PubChem Bioassay (2010) BindingDB Entry DOI: 10.7270/Q2D798W4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50242280 ((2S,3S,4S,5R,6S)-6-(2-(3,4-dihydroxyphenyl)-5,7-di...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Calabria Curated by ChEMBL | Assay Description Inhibition of ACE in rabbit lung assessed as decrease in dansylglycine concentration after 5 mins by HPLC analysis | Bioorg Med Chem Lett 20: 1990-3 (2010) Article DOI: 10.1016/j.bmcl.2010.01.113 BindingDB Entry DOI: 10.7270/Q2G73DW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50242280 ((2S,3S,4S,5R,6S)-6-(2-(3,4-dihydroxyphenyl)-5,7-di...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
India Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of rabbit lung ACE assessed as reduction in hippuryl-histidyl-leucine substrate by colorimetric assay | Bioorg Med Chem 19: 4772-81 (2011) Article DOI: 10.1016/j.bmc.2011.06.085 BindingDB Entry DOI: 10.7270/Q27M089B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||