

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50337661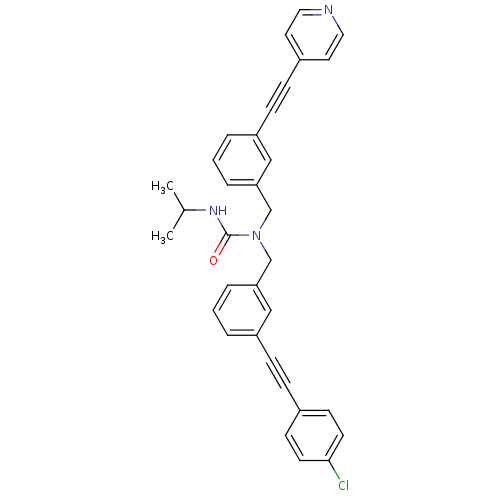 (1-(3-((4-chlorophenyl)ethynyl)benzyl)-3-isopropyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of mPGES1 | Bioorg Med Chem Lett 21: 1488-92 (2011) Article DOI: 10.1016/j.bmcl.2011.01.006 BindingDB Entry DOI: 10.7270/Q2XS5VNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50337661 (1-(3-((4-chlorophenyl)ethynyl)benzyl)-3-isopropyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of mPGES1 in IL-1beta treated human A549 cell microsome assessed as inhibition of PGE2 production after 1 min in presence of 50% FBS | Bioorg Med Chem Lett 21: 1488-92 (2011) Article DOI: 10.1016/j.bmcl.2011.01.006 BindingDB Entry DOI: 10.7270/Q2XS5VNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50337661 (1-(3-((4-chlorophenyl)ethynyl)benzyl)-3-isopropyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of mPGES1 | Bioorg Med Chem Lett 21: 1488-92 (2011) Article DOI: 10.1016/j.bmcl.2011.01.006 BindingDB Entry DOI: 10.7270/Q2XS5VNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50337661 (1-(3-((4-chlorophenyl)ethynyl)benzyl)-3-isopropyl-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of PGF synthase in IL1-beta treated human A549 cell microsome assessed as inhibition of PGF2alpha production after 1 min in presence of 50... | Bioorg Med Chem Lett 21: 1488-92 (2011) Article DOI: 10.1016/j.bmcl.2011.01.006 BindingDB Entry DOI: 10.7270/Q2XS5VNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (Homo sapiens (Human)) | BDBM50337661 (1-(3-((4-chlorophenyl)ethynyl)benzyl)-3-isopropyl-...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research Curated by ChEMBL | Assay Description Inhibition of TX synthase in LPS-stimulated human whole blood assessed as inhibition of TXB2 production | Bioorg Med Chem Lett 21: 1488-92 (2011) Article DOI: 10.1016/j.bmcl.2011.01.006 BindingDB Entry DOI: 10.7270/Q2XS5VNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||