

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM50402074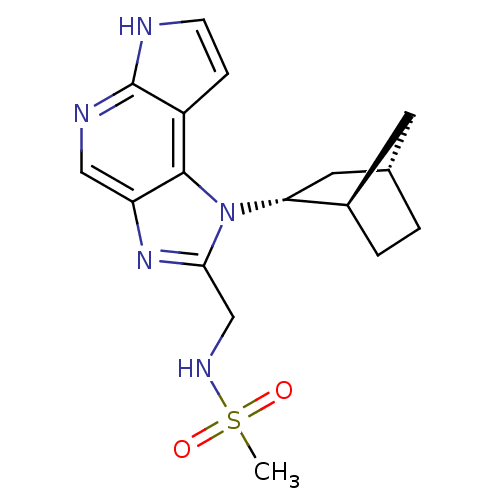 (CHEMBL2206059 | US10112907, Example 00024 | US1076...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The assays were performed in 384-well, low volume microtiter assay plates in a final reaction volume of 9 ul. Dose-response curves were generated by ... | US Patent US10766894 (2020) BindingDB Entry DOI: 10.7270/Q2TX3JDH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase JAK1 [866-1154] (Homo sapiens (Human)) | BDBM50402074 (CHEMBL2206059 | US10112907, Example 00024 | US1076...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assays were performed in 384-well, low volume microtiter assay plates in a final reaction volume of 9 ul. Dose-response curves were generated by ... | Citation and Details BindingDB Entry DOI: 10.7270/Q20K2CRN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase JAK1 [866-1154] (Homo sapiens (Human)) | BDBM50402074 (CHEMBL2206059 | US10112907, Example 00024 | US1076...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | n/a |
NOVARTIS AG US Patent | Assay Description A kinase selectivity panel which measures substrate peptide phosphorylation was set-up for recombinant human Jak1 (aa 866-1154), Jak2 (aa808-1132), J... | US Patent US10112907 (2018) BindingDB Entry DOI: 10.7270/Q2FX7CHW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 [888-1187] (Homo sapiens (Human)) | BDBM50402074 (CHEMBL2206059 | US10112907, Example 00024 | US1076...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assays were performed in 384-well, low volume microtiter assay plates in a final reaction volume of 9 ul. Dose-response curves were generated by ... | Citation and Details BindingDB Entry DOI: 10.7270/Q20K2CRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM50402074 (CHEMBL2206059 | US10112907, Example 00024 | US1076...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The assays were performed in 384-well, low volume microtiter assay plates in a final reaction volume of 9 ul. Dose-response curves were generated by ... | US Patent US10766894 (2020) BindingDB Entry DOI: 10.7270/Q2TX3JDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 [888-1187] (Homo sapiens (Human)) | BDBM50402074 (CHEMBL2206059 | US10112907, Example 00024 | US1076...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 140 | n/a | n/a | n/a | n/a | 7.5 | n/a |
NOVARTIS AG US Patent | Assay Description A kinase selectivity panel which measures substrate peptide phosphorylation was set-up for recombinant human Jak1 (aa 866-1154), Jak2 (aa808-1132), J... | US Patent US10112907 (2018) BindingDB Entry DOI: 10.7270/Q2FX7CHW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 [808-1132] (Homo sapiens (Human)) | BDBM50402074 (CHEMBL2206059 | US10112907, Example 00024 | US1076...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 190 | n/a | n/a | n/a | n/a | 7.5 | n/a |
NOVARTIS AG US Patent | Assay Description A kinase selectivity panel which measures substrate peptide phosphorylation was set-up for recombinant human Jak1 (aa 866-1154), Jak2 (aa808-1132), J... | US Patent US10112907 (2018) BindingDB Entry DOI: 10.7270/Q2FX7CHW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 [808-1132] (Homo sapiens (Human)) | BDBM50402074 (CHEMBL2206059 | US10112907, Example 00024 | US1076...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assays were performed in 384-well, low volume microtiter assay plates in a final reaction volume of 9 ul. Dose-response curves were generated by ... | Citation and Details BindingDB Entry DOI: 10.7270/Q20K2CRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50402074 (CHEMBL2206059 | US10112907, Example 00024 | US1076...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The assays were performed in 384-well, low volume microtiter assay plates in a final reaction volume of 9 ul. Dose-response curves were generated by ... | US Patent US10766894 (2020) BindingDB Entry DOI: 10.7270/Q2TX3JDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 [811-1124] (Homo sapiens (Human)) | BDBM50402074 (CHEMBL2206059 | US10112907, Example 00024 | US1076...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 950 | n/a | n/a | n/a | n/a | 7.5 | n/a |
NOVARTIS AG US Patent | Assay Description A kinase selectivity panel which measures substrate peptide phosphorylation was set-up for recombinant human Jak1 (aa 866-1154), Jak2 (aa808-1132), J... | US Patent US10112907 (2018) BindingDB Entry DOI: 10.7270/Q2FX7CHW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 [811-1124] (Homo sapiens (Human)) | BDBM50402074 (CHEMBL2206059 | US10112907, Example 00024 | US1076...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assays were performed in 384-well, low volume microtiter assay plates in a final reaction volume of 9 ul. Dose-response curves were generated by ... | Citation and Details BindingDB Entry DOI: 10.7270/Q20K2CRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50402074 (CHEMBL2206059 | US10112907, Example 00024 | US1076...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The assays were performed in 384-well, low volume microtiter assay plates in a final reaction volume of 9 ul. Dose-response curves were generated by ... | US Patent US10766894 (2020) BindingDB Entry DOI: 10.7270/Q2TX3JDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50402074 (CHEMBL2206059 | US10112907, Example 00024 | US1076...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of CYP3A4 | Bioorg Med Chem Lett 22: 7627-33 (2012) Article DOI: 10.1016/j.bmcl.2012.10.008 BindingDB Entry DOI: 10.7270/Q2P55PPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50402074 (CHEMBL2206059 | US10112907, Example 00024 | US1076...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of CYP2C9 | Bioorg Med Chem Lett 22: 7627-33 (2012) Article DOI: 10.1016/j.bmcl.2012.10.008 BindingDB Entry DOI: 10.7270/Q2P55PPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50402074 (CHEMBL2206059 | US10112907, Example 00024 | US1076...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of CYP2C19 | Bioorg Med Chem Lett 22: 7627-33 (2012) Article DOI: 10.1016/j.bmcl.2012.10.008 BindingDB Entry DOI: 10.7270/Q2P55PPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50402074 (CHEMBL2206059 | US10112907, Example 00024 | US1076...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of CYP2D6 | Bioorg Med Chem Lett 22: 7627-33 (2012) Article DOI: 10.1016/j.bmcl.2012.10.008 BindingDB Entry DOI: 10.7270/Q2P55PPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50402074 (CHEMBL2206059 | US10112907, Example 00024 | US1076...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of CYP1A2 | Bioorg Med Chem Lett 22: 7627-33 (2012) Article DOI: 10.1016/j.bmcl.2012.10.008 BindingDB Entry DOI: 10.7270/Q2P55PPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||