

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glycogen phosphorylase, muscle form (Oryctolagus cuniculus (rabbit)) | BDBM50016703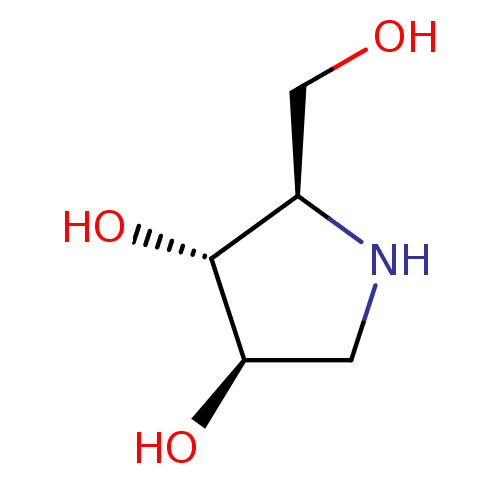 (2-Hydroxymethyl-pyrrolidine-3,4-diol | BDBM5003148...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Claude Bernard Lyon 1 Curated by ChEMBL | Assay Description Inhibition of rabbit muscle glycogen phosphorylase using tritium-labeled glycogen as substrate by scintillation counter | Eur J Med Chem 108: 444-54 (2016) Article DOI: 10.1016/j.ejmech.2015.12.004 BindingDB Entry DOI: 10.7270/Q2QR500M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50016703 (2-Hydroxymethyl-pyrrolidine-3,4-diol | BDBM5003148...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novo Nordisk A/S Curated by ChEMBL | Assay Description Inhibitory activity against pig Liver glycogen phosphorylase a | J Med Chem 47: 3537-45 (2004) Article DOI: 10.1021/jm031121n BindingDB Entry DOI: 10.7270/Q2HM5977 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-mannosidase 2 (Rattus norvegicus) | BDBM50016703 (2-Hydroxymethyl-pyrrolidine-3,4-diol | BDBM5003148...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokuriku University Curated by ChEMBL | Assay Description Competitive Inhibitory activity against Golgi Alpha-mannosidase II | J Med Chem 38: 2349-56 (1995) BindingDB Entry DOI: 10.7270/Q2N878T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50016703 (2-Hydroxymethyl-pyrrolidine-3,4-diol | BDBM5003148...) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | 1.15E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||