 Found 15 hits of ki for monomerid = 50031472
Found 15 hits of ki for monomerid = 50031472 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carbonic anhydrase 4
(Homo sapiens (Human)) | BDBM50031472
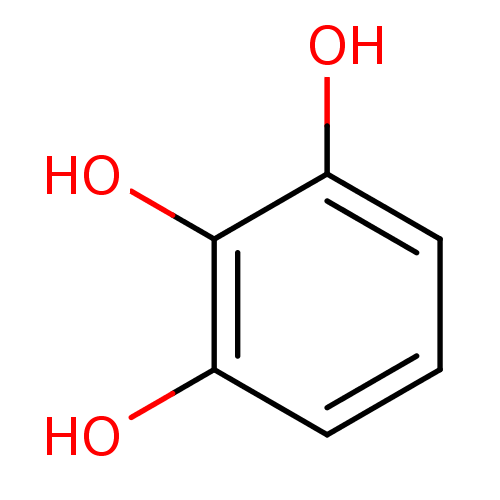
(1,2,3-Trihydroxybenzene, XIV | CHEMBL307145 | Pyro...)Show InChI InChI=1S/C6H6O3/c7-4-2-1-3-5(8)6(4)9/h1-3,7-9H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Çeçen University
| Assay Description
Kinetic studies were performed using the esterase activity method, with 4-nitrophenyl acetate (NPA) as substrate. |
J Enzyme Inhib Med Chem 27: 880-5 (2012)
Article DOI: 10.3109/14756366.2011.637202
BindingDB Entry DOI: 10.7270/Q2VH5MRR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 6
(Homo sapiens (Human)) | BDBM50031472

(1,2,3-Trihydroxybenzene, XIV | CHEMBL307145 | Pyro...)Show InChI InChI=1S/C6H6O3/c7-4-2-1-3-5(8)6(4)9/h1-3,7-9H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gumushane University
Curated by ChEMBL
| Assay Description
Inhibition of human CA6 using 4-nitrophenylacetate substrate by esterase assay |
Bioorg Med Chem Lett 21: 4259-62 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.071
BindingDB Entry DOI: 10.7270/Q2PC32Q0 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50031472

(1,2,3-Trihydroxybenzene, XIV | CHEMBL307145 | Pyro...)Show InChI InChI=1S/C6H6O3/c7-4-2-1-3-5(8)6(4)9/h1-3,7-9H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Çeçen University
| Assay Description
Kinetic studies were performed using the esterase activity method, with 4-nitrophenyl acetate (NPA) as substrate. |
J Enzyme Inhib Med Chem 27: 880-5 (2012)
Article DOI: 10.3109/14756366.2011.637202
BindingDB Entry DOI: 10.7270/Q2VH5MRR |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50031472

(1,2,3-Trihydroxybenzene, XIV | CHEMBL307145 | Pyro...)Show InChI InChI=1S/C6H6O3/c7-4-2-1-3-5(8)6(4)9/h1-3,7-9H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gumushane University
Curated by ChEMBL
| Assay Description
Inhibition of human CA2 using 4-nitrophenylacetate substrate by esterase assay |
Bioorg Med Chem Lett 21: 4259-62 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.071
BindingDB Entry DOI: 10.7270/Q2PC32Q0 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50031472

(1,2,3-Trihydroxybenzene, XIV | CHEMBL307145 | Pyro...)Show InChI InChI=1S/C6H6O3/c7-4-2-1-3-5(8)6(4)9/h1-3,7-9H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | 2.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 5
(Homo sapiens (Human)) | BDBM50031472

(1,2,3-Trihydroxybenzene, XIV | CHEMBL307145 | Pyro...)Show InChI InChI=1S/C6H6O3/c7-4-2-1-3-5(8)6(4)9/h1-3,7-9H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | 3.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50031472

(1,2,3-Trihydroxybenzene, XIV | CHEMBL307145 | Pyro...)Show InChI InChI=1S/C6H6O3/c7-4-2-1-3-5(8)6(4)9/h1-3,7-9H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | 3.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase
(Dicentrarchus labrax) | BDBM50031472

(1,2,3-Trihydroxybenzene, XIV | CHEMBL307145 | Pyro...)Show InChI InChI=1S/C6H6O3/c7-4-2-1-3-5(8)6(4)9/h1-3,7-9H | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 4.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gumushane University
Curated by ChEMBL
| Assay Description
Inhibition of Dicentrarchus labrax CA using 4-nitrophenylacetate substrate by esterase assay |
Bioorg Med Chem Lett 21: 4259-62 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.071
BindingDB Entry DOI: 10.7270/Q2PC32Q0 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50031472

(1,2,3-Trihydroxybenzene, XIV | CHEMBL307145 | Pyro...)Show InChI InChI=1S/C6H6O3/c7-4-2-1-3-5(8)6(4)9/h1-3,7-9H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | 4.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50031472

(1,2,3-Trihydroxybenzene, XIV | CHEMBL307145 | Pyro...)Show InChI InChI=1S/C6H6O3/c7-4-2-1-3-5(8)6(4)9/h1-3,7-9H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | 6.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 6
(Homo sapiens (Human)) | BDBM50031472

(1,2,3-Trihydroxybenzene, XIV | CHEMBL307145 | Pyro...)Show InChI InChI=1S/C6H6O3/c7-4-2-1-3-5(8)6(4)9/h1-3,7-9H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 6.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Çeçen University
| Assay Description
Kinetic studies were performed using the esterase activity method, with 4-nitrophenyl acetate (NPA) as substrate. |
J Enzyme Inhib Med Chem 27: 880-5 (2012)
Article DOI: 10.3109/14756366.2011.637202
BindingDB Entry DOI: 10.7270/Q2VH5MRR |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50031472

(1,2,3-Trihydroxybenzene, XIV | CHEMBL307145 | Pyro...)Show InChI InChI=1S/C6H6O3/c7-4-2-1-3-5(8)6(4)9/h1-3,7-9H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | 7.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50031472

(1,2,3-Trihydroxybenzene, XIV | CHEMBL307145 | Pyro...)Show InChI InChI=1S/C6H6O3/c7-4-2-1-3-5(8)6(4)9/h1-3,7-9H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 7.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agri Ibrahim Çeçen University
| Assay Description
Kinetic studies were performed using the esterase activity method, with 4-nitrophenyl acetate (NPA) as substrate. |
J Enzyme Inhib Med Chem 27: 880-5 (2012)
Article DOI: 10.3109/14756366.2011.637202
BindingDB Entry DOI: 10.7270/Q2VH5MRR |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50031472

(1,2,3-Trihydroxybenzene, XIV | CHEMBL307145 | Pyro...)Show InChI InChI=1S/C6H6O3/c7-4-2-1-3-5(8)6(4)9/h1-3,7-9H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 7.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gumushane University
Curated by ChEMBL
| Assay Description
Inhibition of human CA1 using 4-nitrophenylacetate substrate by esterase assay |
Bioorg Med Chem Lett 21: 4259-62 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.071
BindingDB Entry DOI: 10.7270/Q2PC32Q0 |
More data for this
Ligand-Target Pair | |
Prolyl 4-hydroxylase subunit alpha-1
(Gallus gallus (Chicken)) | BDBM50031472

(1,2,3-Trihydroxybenzene, XIV | CHEMBL307145 | Pyro...)Show InChI InChI=1S/C6H6O3/c7-4-2-1-3-5(8)6(4)9/h1-3,7-9H | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oulu
| Assay Description
Inhibition assay using procollagen-prolin, 2-oxoglutarate 4-dioxygenase. |
J Biol Chem 261: 7819-23 (1986)
BindingDB Entry DOI: 10.7270/Q2SJ1J6Z |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data