

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chymase (Homo sapiens (Human)) | BDBM50068898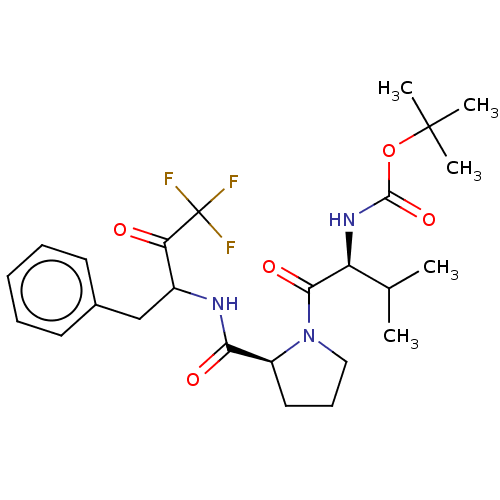 (BDBM50281588 | CHEMBL147013 | {1-[2-(1-Benzyl-3,3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50068898 (BDBM50281588 | CHEMBL147013 | {1-[2-(1-Benzyl-3,3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 113 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against Chymotrypsinogen | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin G (Homo sapiens (Human)) | BDBM50068898 (BDBM50281588 | CHEMBL147013 | {1-[2-(1-Benzyl-3,3,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for its potency to inhibit human Cathepsin G | Bioorg Med Chem Lett 3: 525-530 (1993) Article DOI: 10.1016/S0960-894X(01)81220-8 BindingDB Entry DOI: 10.7270/Q23778NF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||