

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gastrin/cholecystokinin type B receptor (MOUSE) | BDBM50230678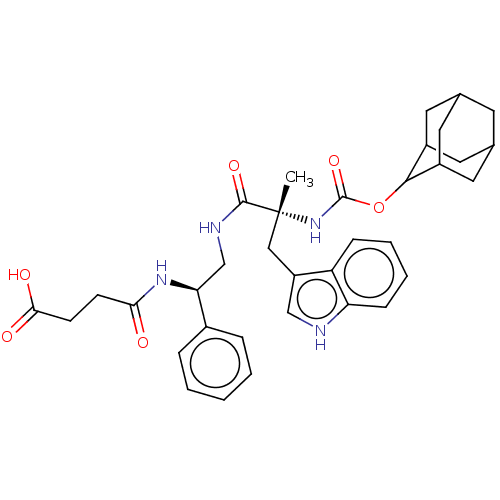 (CHEMBL287735) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Neuroscience Research Centre Curated by ChEMBL | Assay Description Cholecystokinin type B receptor binding assay performed on homogenized cerebral cortex from male mouse | J Med Chem 35: 1572-7 (1992) BindingDB Entry DOI: 10.7270/Q26H4KM8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||