

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433477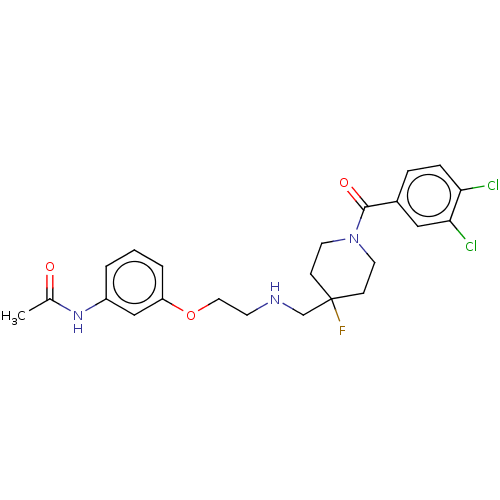 (US10562853, Compound 3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0537 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]8-OH-DPAT from human 5-HT1A receptor stably expressed in CHO-K1 cell membranes measured after 60 mins by Microbeta2 scintillation... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00814 BindingDB Entry DOI: 10.7270/Q24T6NZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433477 (US10562853, Compound 3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NEUROLIXIS; UNIVERSITE JAGELLONE US Patent | Assay Description 5-HT1A: Radioligand binding was performed using membranes from CHO-K1 cells stably transfected with the human 5-HT1A receptor. All assays were carrie... | US Patent US10562853 (2020) BindingDB Entry DOI: 10.7270/Q29Z979D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433477 (US10562853, Compound 3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.0251 | n/a | n/a | n/a | n/a |
TBA | Assay Description Biased agonist activity at Gal4-VP16 transcription factor linked human 5-HT1A receptor expressed in human U2OS cells assessed as induction of beta-ar... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00814 BindingDB Entry DOI: 10.7270/Q24T6NZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433477 (US10562853, Compound 3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.0661 | n/a | n/a | n/a | n/a |
TBA | Assay Description Biased agonist activity at human 5-HT1A receptor stably expressed in CHO-K1 cells assessed as inhibition of forskolin-induced cAMP accumulation after... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00814 BindingDB Entry DOI: 10.7270/Q24T6NZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433477 (US10562853, Compound 3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 44 | n/a | n/a | n/a | n/a |
TBA | Assay Description Biased agonist activity at human 5-HT1A receptor stably expressed in CHO-K1 cells co-expressing Galpha16/GPCR assessed as increase in calcium mobiliz... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00814 BindingDB Entry DOI: 10.7270/Q24T6NZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM433477 (US10562853, Compound 3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.589 | n/a | n/a | n/a | n/a |
TBA | Assay Description Biased agonist activity at human 5-HT1A receptor stably expressed in CHO-K1 cells assessed as increase in ERK1/2 phosphorylation after 15 mins by Alp... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00814 BindingDB Entry DOI: 10.7270/Q24T6NZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||