 Found 9 hits Enz. Inhib. hit(s) with Target = '5-hydroxytryptamine receptor 2A' and Ligand = 'BDBM50024204'
Found 9 hits Enz. Inhib. hit(s) with Target = '5-hydroxytryptamine receptor 2A' and Ligand = 'BDBM50024204' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50024204
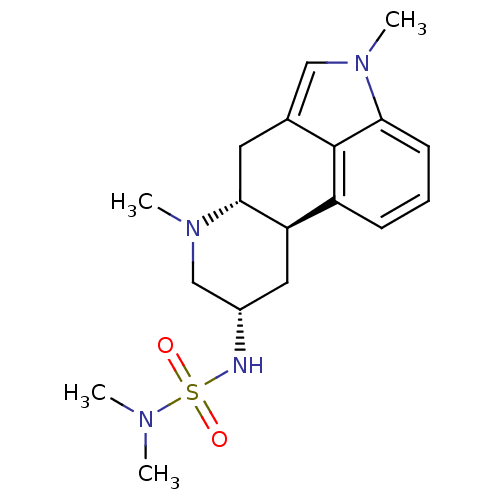
(1H-imidazo[4,5-c]pyridine derivative | 2N-[4,7-dim...)Show SMILES CN(C)S(=O)(=O)N[C@H]1C[C@H]2[C@@H](Cc3cn(C)c4cccc2c34)N(C)C1 Show InChI InChI=1S/C18H26N4O2S/c1-20(2)25(23,24)19-13-9-15-14-6-5-7-16-18(14)12(10-21(16)3)8-17(15)22(4)11-13/h5-7,10,13,15,17,19H,8-9,11H2,1-4H3/t13-,15+,17+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 260: 1361-5 (1992)
BindingDB Entry DOI: 10.7270/Q2513WPC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50024204

(1H-imidazo[4,5-c]pyridine derivative | 2N-[4,7-dim...)Show SMILES CN(C)S(=O)(=O)N[C@H]1C[C@H]2[C@@H](Cc3cn(C)c4cccc2c34)N(C)C1 Show InChI InChI=1S/C18H26N4O2S/c1-20(2)25(23,24)19-13-9-15-14-6-5-7-16-18(14)12(10-21(16)3)8-17(15)22(4)11-13/h5-7,10,13,15,17,19H,8-9,11H2,1-4H3/t13-,15+,17+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University
Curated by PDSP Ki Database
| |
J Neurosci 9: 3482-90 (1989)
BindingDB Entry DOI: 10.7270/Q2FX77Z2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50024204

(1H-imidazo[4,5-c]pyridine derivative | 2N-[4,7-dim...)Show SMILES CN(C)S(=O)(=O)N[C@H]1C[C@H]2[C@@H](Cc3cn(C)c4cccc2c34)N(C)C1 Show InChI InChI=1S/C18H26N4O2S/c1-20(2)25(23,24)19-13-9-15-14-6-5-7-16-18(14)12(10-21(16)3)8-17(15)22(4)11-13/h5-7,10,13,15,17,19H,8-9,11H2,1-4H3/t13-,15+,17+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
Neuropharmacology 36: 621-9 (1997)
Article DOI: 10.1016/s0028-3908(97)00049-x
BindingDB Entry DOI: 10.7270/Q2NV9GSN |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50024204

(1H-imidazo[4,5-c]pyridine derivative | 2N-[4,7-dim...)Show SMILES CN(C)S(=O)(=O)N[C@H]1C[C@H]2[C@@H](Cc3cn(C)c4cccc2c34)N(C)C1 Show InChI InChI=1S/C18H26N4O2S/c1-20(2)25(23,24)19-13-9-15-14-6-5-7-16-18(14)12(10-21(16)3)8-17(15)22(4)11-13/h5-7,10,13,15,17,19H,8-9,11H2,1-4H3/t13-,15+,17+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 265: 1272-9 (1993)
BindingDB Entry DOI: 10.7270/Q2833QJ4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50024204

(1H-imidazo[4,5-c]pyridine derivative | 2N-[4,7-dim...)Show SMILES CN(C)S(=O)(=O)N[C@H]1C[C@H]2[C@@H](Cc3cn(C)c4cccc2c34)N(C)C1 Show InChI InChI=1S/C18H26N4O2S/c1-20(2)25(23,24)19-13-9-15-14-6-5-7-16-18(14)12(10-21(16)3)8-17(15)22(4)11-13/h5-7,10,13,15,17,19H,8-9,11H2,1-4H3/t13-,15+,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 19.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
Neuropharmacology 36: 621-9 (1997)
Article DOI: 10.1016/s0028-3908(97)00049-x
BindingDB Entry DOI: 10.7270/Q2NV9GSN |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50024204

(1H-imidazo[4,5-c]pyridine derivative | 2N-[4,7-dim...)Show SMILES CN(C)S(=O)(=O)N[C@H]1C[C@H]2[C@@H](Cc3cn(C)c4cccc2c34)N(C)C1 Show InChI InChI=1S/C18H26N4O2S/c1-20(2)25(23,24)19-13-9-15-14-6-5-7-16-18(14)12(10-21(16)3)8-17(15)22(4)11-13/h5-7,10,13,15,17,19H,8-9,11H2,1-4H3/t13-,15+,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-ketanserin from human cloned 5-hydroxytryptamine 2A receptor expressed in CHO-K1 cells. |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2HD7XSJ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Monkey) | BDBM50024204

(1H-imidazo[4,5-c]pyridine derivative | 2N-[4,7-dim...)Show SMILES CN(C)S(=O)(=O)N[C@H]1C[C@H]2[C@@H](Cc3cn(C)c4cccc2c34)N(C)C1 Show InChI InChI=1S/C18H26N4O2S/c1-20(2)25(23,24)19-13-9-15-14-6-5-7-16-18(14)12(10-21(16)3)8-17(15)22(4)11-13/h5-7,10,13,15,17,19H,8-9,11H2,1-4H3/t13-,15+,17+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 44.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 265: 1272-9 (1993)
BindingDB Entry DOI: 10.7270/Q2833QJ4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(PIG) | BDBM50024204

(1H-imidazo[4,5-c]pyridine derivative | 2N-[4,7-dim...)Show SMILES CN(C)S(=O)(=O)N[C@H]1C[C@H]2[C@@H](Cc3cn(C)c4cccc2c34)N(C)C1 Show InChI InChI=1S/C18H26N4O2S/c1-20(2)25(23,24)19-13-9-15-14-6-5-7-16-18(14)12(10-21(16)3)8-17(15)22(4)11-13/h5-7,10,13,15,17,19H,8-9,11H2,1-4H3/t13-,15+,17+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 54.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 265: 1272-9 (1993)
BindingDB Entry DOI: 10.7270/Q2833QJ4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50024204

(1H-imidazo[4,5-c]pyridine derivative | 2N-[4,7-dim...)Show SMILES CN(C)S(=O)(=O)N[C@H]1C[C@H]2[C@@H](Cc3cn(C)c4cccc2c34)N(C)C1 Show InChI InChI=1S/C18H26N4O2S/c1-20(2)25(23,24)19-13-9-15-14-6-5-7-16-18(14)12(10-21(16)3)8-17(15)22(4)11-13/h5-7,10,13,15,17,19H,8-9,11H2,1-4H3/t13-,15+,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Alberta
Curated by PDSP Ki Database
| |
Neuropharmacology 33: 275-317 (1994)
Article DOI: 10.1016/0028-3908(94)90059-0
BindingDB Entry DOI: 10.7270/Q2M043X2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data