

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50181836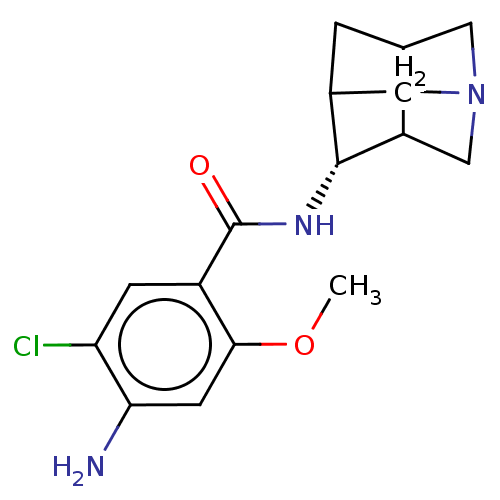 (4-Amino-N-(1-aza-tricyclo[3.3.1.0*3,7*]non-4-yl)-5...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]GR-113808 from 5HT4 receptor in guinea pig striatum | J Med Chem 49: 1125-39 (2006) Article DOI: 10.1021/jm0509501 BindingDB Entry DOI: 10.7270/Q2W096Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (RAT) | BDBM50181836 (4-Amino-N-(1-aza-tricyclo[3.3.1.0*3,7*]non-4-yl)-5...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 51 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonism at 5HT4 receptor in rat tunica muscularis mucosa | J Med Chem 49: 1125-39 (2006) Article DOI: 10.1021/jm0509501 BindingDB Entry DOI: 10.7270/Q2W096Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (RAT) | BDBM50181836 (4-Amino-N-(1-aza-tricyclo[3.3.1.0*3,7*]non-4-yl)-5...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | n/a | n/a | 51 | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the agonistic activity towards 5-hydroxytryptamine 4 receptor using the rat tunica muscularis mucosae (TMM) esophagus stri... | Bioorg Med Chem Lett 2: 1613-1618 (1992) Article DOI: 10.1016/S0960-894X(00)80441-2 BindingDB Entry DOI: 10.7270/Q2J67HFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||