

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50456116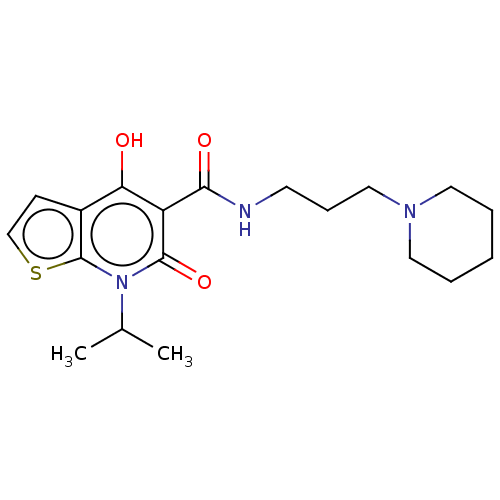 (Prx-03140) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 58 | n/a | n/a | n/a | n/a |
Suven Life Sciences Limited Curated by ChEMBL | Assay Description Agonist activity at recombinant human 5-HT4E receptor expressed in CHO cells assessed as induction of c-AMP accumulation after 4 hrs by luciferase re... | J Med Chem 61: 4993-5008 (2018) Article DOI: 10.1021/acs.jmedchem.8b00457 BindingDB Entry DOI: 10.7270/Q21R6T32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50456116 (Prx-03140) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a |
Suven Life Sciences Limited Curated by ChEMBL | Assay Description Agonist activity at 5-HT4E receptor (unknown origin) | J Med Chem 61: 4993-5008 (2018) Article DOI: 10.1021/acs.jmedchem.8b00457 BindingDB Entry DOI: 10.7270/Q21R6T32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50456116 (Prx-03140) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a | |
TBA | Assay Description The compound was tested for beta-adrenergic activity against Beta-1 adrenergic receptor from guinea pig right atria | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50456116 (Prx-03140) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd. Curated by ChEMBL | Assay Description Agonist activity at recombinant human 5HT4e receptor expressed in African green monkey COS7 cells assessed as cAMP level after 30 mins by HTRF assay | Eur J Med Chem 103: 289-301 (2015) Article DOI: 10.1016/j.ejmech.2015.08.051 BindingDB Entry DOI: 10.7270/Q20C4ZST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50456116 (Prx-03140) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | n/a | n/a | 58 | n/a | n/a | n/a | n/a | |
TBA | Assay Description In vitro inhibition of angiotensin I converting enzyme in rabbit lung with hippuryl-histidyl-leucine as substrate | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50456116 (Prx-03140) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 58 | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd. Curated by ChEMBL | Assay Description Agonist activity at human 5HT4e receptor expressed in CHO cells assessed as cAMP level after 4 hrs by luciferase reporter gene assay | Eur J Med Chem 103: 289-301 (2015) Article DOI: 10.1016/j.ejmech.2015.08.051 BindingDB Entry DOI: 10.7270/Q20C4ZST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||