

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50104969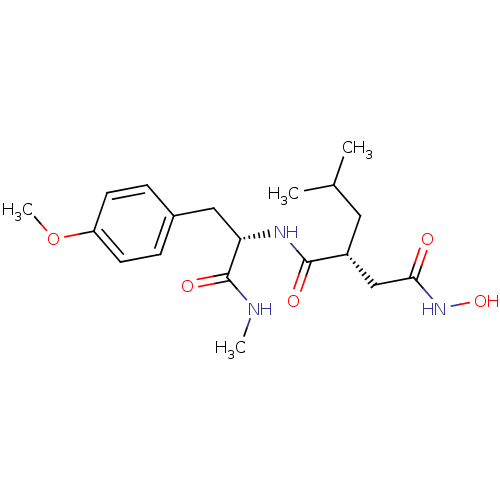 ((R)-N*4*-Hydroxy-2-isobutyl-N*1*-[(S)-2-(4-methoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibition of matrix metalloprotease-2 | J Med Chem 44: 3347-50 (2001) BindingDB Entry DOI: 10.7270/Q2057F7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50104969 ((R)-N*4*-Hydroxy-2-isobutyl-N*1*-[(S)-2-(4-methoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article | <10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Matrix metalloproteinase-2 (MMP-2) | Bioorg Med Chem Lett 7: 2331-2336 (1997) Article DOI: 10.1016/S0960-894X(97)00182-0 BindingDB Entry DOI: 10.7270/Q2TH8MQ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50104969 ((R)-N*4*-Hydroxy-2-isobutyl-N*1*-[(S)-2-(4-methoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human matrix metalloprotease-2 (MMP-2) | J Med Chem 46: 3514-25 (2003) Article DOI: 10.1021/jm0308038 BindingDB Entry DOI: 10.7270/Q24F1Q3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||