

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM10625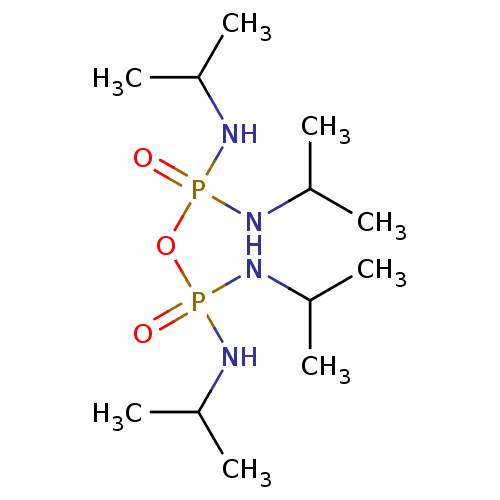 (({[bis(propan-2-ylamino)phosphoryl]oxy}(propan-2-y...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Trinity College Curated by ChEMBL | Assay Description Inhibition of electric eel AchE by Ellman's method | J Med Chem 51: 6400-9 (2008) Article DOI: 10.1021/jm800564y BindingDB Entry DOI: 10.7270/Q2RV0NJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM10625 (({[bis(propan-2-ylamino)phosphoryl]oxy}(propan-2-y...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Natural Products Applications and Research Technologies Curated by ChEMBL | Assay Description Inhibition of electric eel AChE incubated for 15 mins by Ellman's method | J Nat Prod 82: 2627-2637 (2019) Article DOI: 10.1021/acs.jnatprod.9b00563 BindingDB Entry DOI: 10.7270/Q2028W03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM10625 (({[bis(propan-2-ylamino)phosphoryl]oxy}(propan-2-y...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Natural Products Applications and Research Technologies Curated by ChEMBL | Assay Description Inhibition of electric eel AChE incubated for 15 mins by Ellman's method | J Nat Prod 82: 2627-2637 (2019) Article DOI: 10.1021/acs.jnatprod.9b00563 BindingDB Entry DOI: 10.7270/Q2028W03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10625 (({[bis(propan-2-ylamino)phosphoryl]oxy}(propan-2-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 2174-85 (2006) Article DOI: 10.1021/jm050578p BindingDB Entry DOI: 10.7270/Q2S75DJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||