

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50175500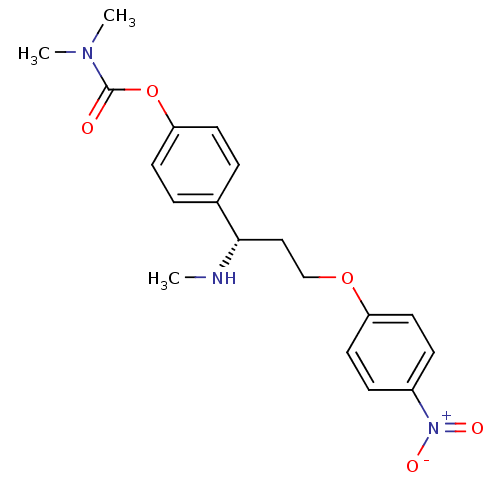 ((S)-4-(1-(methylamino)-3-(4-nitrophenoxy)propyl)ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against acetylcholinesterase | J Med Chem 48: 6523-43 (2005) Article DOI: 10.1021/jm058225d BindingDB Entry DOI: 10.7270/Q2SF2WZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50175500 ((S)-4-(1-(methylamino)-3-(4-nitrophenoxy)propyl)ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Inhibition of human AchE | J Med Chem 51: 347-72 (2008) Article DOI: 10.1021/jm7009364 BindingDB Entry DOI: 10.7270/Q25B039W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50175500 ((S)-4-(1-(methylamino)-3-(4-nitrophenoxy)propyl)ph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114606 BindingDB Entry DOI: 10.7270/Q2FJ2MW1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50175500 ((S)-4-(1-(methylamino)-3-(4-nitrophenoxy)propyl)ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Central Drug Research Institute Curated by ChEMBL | Assay Description Inhibition of mouse brain acetylcholinesterase by Ellman's method | Bioorg Med Chem 27: 895-930 (2019) Article DOI: 10.1016/j.bmc.2019.01.025 BindingDB Entry DOI: 10.7270/Q2N87F3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50175500 ((S)-4-(1-(methylamino)-3-(4-nitrophenoxy)propyl)ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of mouse brain AChE | J Med Chem 62: 8881-8914 (2019) Article DOI: 10.1021/acs.jmedchem.9b00017 BindingDB Entry DOI: 10.7270/Q27084PF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||