

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50280646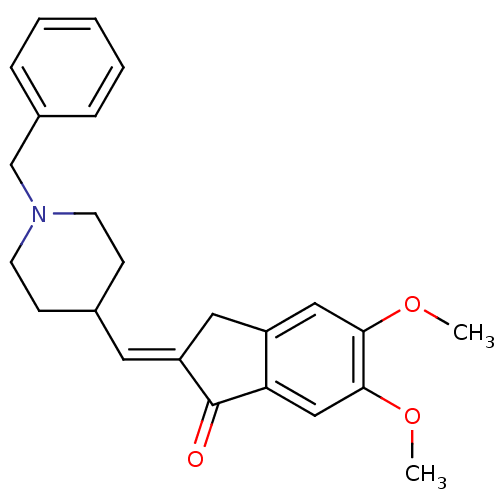 (2-[1-(1-Benzyl-piperidin-4-yl)-meth-(E)-ylidene]-5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi della Magna Gr£cia Viale Europa Curated by ChEMBL | Assay Description Non-competitive inhibition of human erythrocytes AchE using acetylthiocholine iodide as substrate incubated for 20 mins by Lineweaver-Burk plot analy... | ACS Med Chem Lett 7: 470-5 (2016) Article DOI: 10.1021/acsmedchemlett.5b00483 BindingDB Entry DOI: 10.7270/Q2PN97JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50280646 (2-[1-(1-Benzyl-piperidin-4-yl)-meth-(E)-ylidene]-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Acetylcholinesterase activity in mouse brain homogenate | Bioorg Med Chem Lett 2: 871-876 (1992) Article DOI: 10.1016/S0960-894X(00)80547-8 BindingDB Entry DOI: 10.7270/Q21R6QDV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50280646 (2-[1-(1-Benzyl-piperidin-4-yl)-meth-(E)-ylidene]-5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi della Magna Gr£cia Viale Europa Curated by ChEMBL | Assay Description Inhibition of human erythrocytes AchE using acetylthiocholine iodide as substrate incubated for 20 mins by Ellman method | ACS Med Chem Lett 7: 470-5 (2016) Article DOI: 10.1021/acsmedchemlett.5b00483 BindingDB Entry DOI: 10.7270/Q2PN97JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50280646 (2-[1-(1-Benzyl-piperidin-4-yl)-meth-(E)-ylidene]-5...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pretoria Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate by Ellman's method | Eur J Med Chem 127: 671-690 (2017) Article DOI: 10.1016/j.ejmech.2016.10.036 BindingDB Entry DOI: 10.7270/Q2P84F48 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||