

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine kinase (Homo sapiens (Human)) | BDBM50090895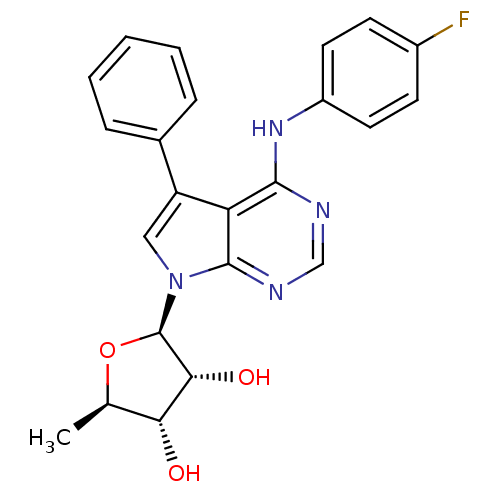 ((2R,3R,4S,5R)-2-[4-(4-Fluoro-phenylamino)-5-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Metabasis Therapeutics Inc Curated by ChEMBL | Assay Description Inhibition of recombinant human adenosine kinase | J Med Chem 43: 2894-905 (2000) BindingDB Entry DOI: 10.7270/Q2SQ8ZMP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50090895 ((2R,3R,4S,5R)-2-[4-(4-Fluoro-phenylamino)-5-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Metabasis Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against recombinant human adenosine kinase | J Med Chem 48: 7808-20 (2005) Article DOI: 10.1021/jm050394a BindingDB Entry DOI: 10.7270/Q22V2FPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50090895 ((2R,3R,4S,5R)-2-[4-(4-Fluoro-phenylamino)-5-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of human adenosine kinase assessed as reduction in conversion of adenosine to AMP | J Med Chem 59: 6860-77 (2016) Article DOI: 10.1021/acs.jmedchem.6b00689 BindingDB Entry DOI: 10.7270/Q2TF01TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Rattus norvegicus (rat)) | BDBM50090895 ((2R,3R,4S,5R)-2-[4-(4-Fluoro-phenylamino)-5-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of Adenosine kinase (AK) | J Med Chem 44: 2133-8 (2001) BindingDB Entry DOI: 10.7270/Q25D8R47 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50090895 ((2R,3R,4S,5R)-2-[4-(4-Fluoro-phenylamino)-5-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Metabasis Therapeutics, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against human recombinant adenosine kinase | J Med Chem 46: 4750-60 (2003) Article DOI: 10.1021/jm030230z BindingDB Entry DOI: 10.7270/Q2FB52B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50090895 ((2R,3R,4S,5R)-2-[4-(4-Fluoro-phenylamino)-5-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 134 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of adenosine phosphorylation in confluent IMR-32 (human neuroblastoma) cells. | J Med Chem 44: 2133-8 (2001) BindingDB Entry DOI: 10.7270/Q25D8R47 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||