

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189515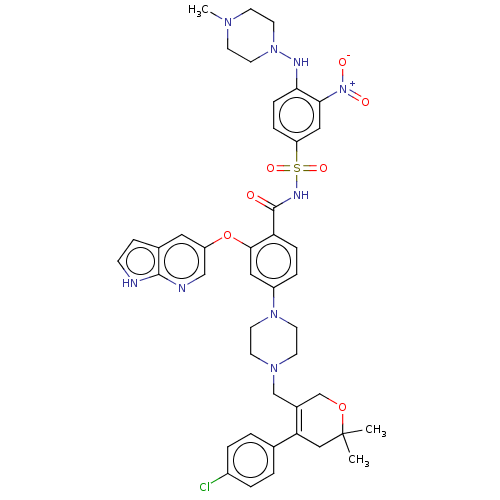 (US10213433, Compound 67 | US11369599, Compound 67 ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <0.0100 | <-62.8 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC.; GENENTECH, INC.; THE WALTER AND ELIZA HALL INSTITUTE OF MEDICAL RESEARCH US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9174982 (2015) BindingDB Entry DOI: 10.7270/Q2RB73D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189515 (US10213433, Compound 67 | US11369599, Compound 67 ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189515 (US10213433, Compound 67 | US11369599, Compound 67 ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibition constant (Ki) for binding of representative compounds to Bcl-2 protein, as determined by a TR-FRET (Time-Resolved Fluorescence-Resonan... | Citation and Details BindingDB Entry DOI: 10.7270/Q21V5J59 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189515 (US10213433, Compound 67 | US11369599, Compound 67 ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description TR-FRET (Time-Resolved Fluorescence-Resonance-Energy-Transfer) assay. | US Patent US10213433 (2019) BindingDB Entry DOI: 10.7270/Q23F4RZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||